Feature Article, Company News
Introduction of Nopol and Nopyl acetate
Feature Article, Company News

| Nopol | Nopyl acetate | |
| Molecular Formula | C11H18O | C13H20O2 |
| Synonyms | (-)-Nopol 35836-73-8 2-((1R,5S)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethanol Nopol, (-)- (1R)-(-)-Nopyl alcohol … |
Nopyl acetate 128-51-8 Nopol acetate Citroviol Lignyl acetate … |
| CAS | 35836-73-8 | 128-51-8 |
| EINECS | 252-744-2 | 204-891-9 |
There are many downstream products of β-Pinene. For example, β-Pinene can be thermally cracked to obtain Myrcene, which can then be catalyzed by HCl to produce Neryl chloride, leading to the production of high-value fragrances such as Linalool, Nerol, or Geraniol. Alternatively, β-Pinene can be catalyzed to produce Geranyl chloride, and eventually yield Geranyl acetone.
Nopol is one of the synthetic routes of downstream flavors of β-Pinene, which can ultimately produce substances such as Homoperillaldehyde, Homoperillyl alcohol, and Nopol Acetate. The synthesis of Nopol mainly includes three methods: ZnCl2-catalyzed method, acid-catalyzed method, and hot-pressing method. a) In the ZnCl2-catalyzed method, Nopol is obtained by the reaction of ZnCl2 catalyzed of 1,3,5-trioxane or Polyoxymethylene (POM) with β-Pinene. This route has more by-products (XIAO Zhuanquan, 1999)1.
b) Acid-catalyzed method, Nopol can be synthesized through the Prins reaction of β-Pinene and 1,3,5-trioxane or POM under the action of solid superacid, formic acid, and acetic acid. c) Hot-pressing method, β-Pinene and formaldehyde are used to produce Nopol through high temperature and high pressure. This method does not easy producing other higher boiling point isomers by-products due to the high temperature and pressure conditions. Currently, the hot-pressing method process is preferred among the three synthetic routes, as it has fewer by-products and higher yield.
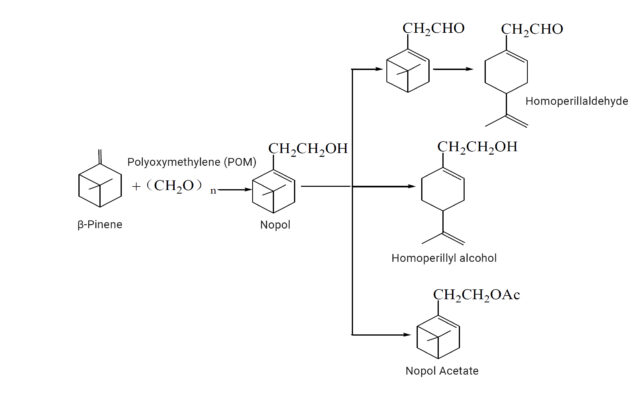
Synthetic route of Nopol and Nopol acetate from beta-pinene
Nopol can be used as a starting material for Pinaverium bromide, a selective gastrointestinal calcium ion antagonist used to treat irritable bowel syndrome. Due to its woody and minty aroma, Nopol is often used as a starting material for flavorings and fragrances. Under Cu-Zn catalysis, it can be thermally decomposed to produce an intermediate (Limonene-7-methanol) with a violet fragrance. This intermediate can be esterified with acetic acid to produce Homoperillyl acetate, or oxidized to produce Homoperillaldehyde.
When Nopol and acetic anhydride or acetic acid undergo esterification reaction, Nopyl acetate can be produced. Nopyl acetate has a sweet and woody sense and is commonly used in cosmetic fragrance formulas, paired with lavender, jasmine, fougee, citrus, bergamot, and orange blossom scents. It has been used as a substitute for Linalyl acetate, showing less sweetness and fruity notes, and more woody character compared to Linalyl acetate (Arctander, 2019)2.
Now, Nopyl acetate has found its role in the flavor and fragrance (F&F) market as a cost-effective fragrance ingredient used in personal care formulations. It is also a good masking agent for masking the chemical odor emitted by compounds in personal care product formulations. Nopyl acetate can also be applied in fuel additive formulations to improve the combustion efficiency of diesel engines (United States Patent No. US20210139799A1, 2019)3.