Industry News, Adhesives & Sealants & Tackifiers
The Benefits of Caprolactone Technology in Reactive Hot Melt Adhesives
Industry News, Adhesives & Sealants & Tackifiers

Feature pic from MarekUsz / iStock / Getty Images
Reactive hot melt adhesives provide advantages in a variety of end-application areas that include textiles, woodworking, construction, automotive, bookbinding, and electronics. A combination of the initial recrystallisation after application with the subsequent polyurethane polymerization gives this type of adhesive platform superior adhesion properties with enhanced chemical and heat resistance compared to traditional non-reactive hot melt adhesives.
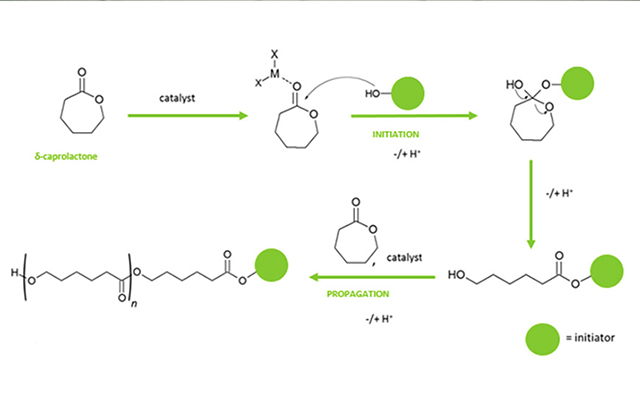
Polycaprolactone is polymerized via a ring opening synthesis route, where caprolactone monomer is ring-opened via an initiator moiety. The initiator moiety is a hydroxyl functionalized molecule, which can be a diol, triol, or tetrol (Figure 1).
Link to ASI to learn full figures/tabels.
The mechanism shown in Figure 1 is the genesis of the specialty products produced, as caprolactone polyols have low acid values (Max 0.25) providing durability in application, low color, and a narrow polydispersity (<1.5).
In reactive hot melt adhesives, polycaprolactone polyols are commonly diols of molecular weight between 2,000 and 4,000 and are used in the soft segments of the polyurethane structure. In addition to polycaprolactone polyols, polycaprolactone thermoplastics are used to enhance the performance of the adhesive. The polycaprolactone thermoplastics provide increased green strength to the adhesive and are used in combination with polyurethane pre-polymers.
A study conducted at Ingevity’s new Innovation Centre in Warrington, UK, has fully characterized the performance of a 4,000 MWt caprolactone diol versus that of a commercially available polyester diol. Table 1 shows the details of the polyols used in the study.
Formulators have a variety of polyol chemistries to select from when considering the desired properties of their polyurethane soft segment. Polyether polyols from 1000-2000 molecular weight are typically used, with polyester chemistry being incorporated to increase the levels of adhesion or to tune crystallinity for control of adhesive open times. The polyols presented in Table 1, and the subsequent data presented in this article, demonstrate the performance benefits achievable with caprolactone polyols produced via a ring opening polymerization.
Reactive polyurethane hot melt adhesives were synthesized with a 3% excess isocyanate content. The formulations were kept simple to allow for a direct comparison between the caprolactone and the polyester diols respectively. The formulations characterized in this study are based on a reaction between a diol and monomeric methylene diphenyl diisocyanate (MDI), with a 3% excess of isocyanate content, to allow for the subsequent reaction between the isocyanate and ambient moisture, converting the final adhesive into a thermoset polymer.
Presented below is the full characterization (viscosity, adhesion, tensile properties, and open time) of two reactive hot melt adhesives, one based on a 4,000 MWt polycaprolactone diol and a second based on a commercially available polyester diol.
Link to ASI to learn full figures/tabels.
A Brookfield Viscometer, using Spindle 27 with a Thermosel, was used to measure the viscosity of the adhesive at application temperature (120-130 ⁰C). Viscosity is an important parameter to consider as it allows the formulator to consider how the adhesive is going to wet the substrate, and on porous substrates, if the adhesive can penetrate into the structure of the substrate.
As shown in Figure 2, the viscosity of the caprolactone-based adhesive was lower than that of the polyester-based adhesive. This is further emphasized with the viscosity being lower, even with a reduction in temperature of 10 ⁰C. As pointed out, this can provide benefits when considering wetting and flow properties of the adhesive. It also provides the option of lowering the application temperature of the adhesive, which can provide energy savings and help to protect sensitive substrates from elevated temperatures.
The lower viscosity of the polycaprolactone is attributed to its ring opening polymerization synthesis route and the control on the polydispersity it brings.
A lap shear method was followed to measure the adhesion between substrates (Fmax, N/mm2) and the failure mechanism was also noted during the test. The adhesive was applied at 120 ⁰C via a hot melt gun and the bonded article was left for 8 days to cure at 23 ⁰C and 50% RH. The substrates under test, polycarbonate (6mm), beechwood (6mm), and aluminum (2mm) were studied with the data presented in Figures 3 and 4.
From Figure 3, it is evident that the performance of the adhesives is comparable on polycarbonate. With beechwood, the caprolactone-based adhesives were consistently producing substrate failures. The beechwood was cracking during the test, whereas in comparison, the polyester-based adhesive gave adhesion failure under the same test conditions. It can be hypothesized that the lower viscosity caprolactone adhesive is wetting and further penetrating the wood to give a more mechanical bond to induce this failure type.
Link to ASI to learn full figures/tabels.
Figure 4 illustrates an increased level of adhesion with the caprolactone-based adhesive in comparison to the polyester-based one. This may provide interest to those formulators working in electronic or automotive applications where adhesion to aluminum is critical to quality.
The mechanical performance of a thin adhesive film (0.5mm) was measured via a tensiometer to dimensions specified in ISO 37:20051. The incorporation of a polyester into a reactive hot melt formulation will not only give benefits in terms of adhesion but will also increase mechanical performance of the cured adhesive film in comparison to polyethers.
It was shown in the study that the caprolactone-based adhesive gave comparable Fmax (MPa) values to polyester, in the region of 24 MPa. The percentage elongation was also consistent between the two candidates with 520% elongation before break. Therefore, it was possible to achieve the best-in-class mechanical performance and maintain low viscosity with the caprolactone-based adhesive.
The open time of the adhesive in this study was defined by applying 0.2 grams of adhesive to a wooden tongue depressor at 120 ⁰C. When the adhesive was unable to wet a second substrate to form a satisfactory bond, the time elapsed was used to define the open time of the adhesive. To understand the thermal behavior of the polyols and the final adhesive film under investigation, differential scanning calorimetry (DSC) was used to measure the glass transition temperature (Tg) and recrystallisation temperature (Tc), shown in Table 2.
The glass transition and recrystallisation temperatures (⁰C) are lower in the caprolactone polyol and subsequent adhesive. This also translates to a longer open time for the caprolactone-based adhesive (7 minutes 30 seconds vs 3 minutes 45 seconds for the polyester adhesive). Extended open time of the caprolactone adhesive has provided scope to investigate the impact of altering the initiator moiety in the ring opening polymerization (see Figure 1). By introducing longer carbon chains, ring structures or aromaticity into the initiator moiety it has been possible to alter the structure property relationship of the final adhesive with the aim of reducing the open time of the adhesive.
Within this study, it was found that the recrystallisation temperature (Tc) of the polyol was the best indicator for subsequent open time tests of the final adhesive. This was demonstrated by Caprolactone initiator C, which has a Tc of 29 ⁰C and a resulting open time of 4 minutes and 45 seconds. This is a reduction of 2 minutes and 45 seconds from the current commercially available caprolactone polyol.
By altering the initiator chemistry, the open time was not only reduced but it was also possible to maintain the enhanced adhesion and lower viscosity benefits that the caprolactone platform provides.
This study has provided supporting data to the strong value proposition of the use of caprolactone technology within reactive hot melt formulations. The caprolactone polyols produce low viscosity reactive hot melt adhesives that enhance processing conditions and increase substrate wetting.
Increased levels of adhesion were measured to both aluminum and beechwood substrates. Mechanical performance of both the polyester and caprolactone were comparable.
The thermal behavior of both the polyols under investigation and the formulated adhesives were investigated via DSC and linked to the open time of adhesive. It was shown that the caprolactone-based adhesives have longer open times than the polyester alternative. Longer open times are of benefit in applications where complex and sensitive substrates are bonded. Innovative strategies have been employed to provide caprolactone polyols that provide reactive hot melt adhesives with shorter open times but maintain the lower viscosity and enhanced adhesion of current caprolactone products.