R&D
Catalytic Upgrading of Extractives to Chemicals: Monoterpenes to “EXICALS”
R&D

Article was published on CHEMICAL REVIEWS, ACS Publications
Evidently, the global demand for oil products is bound to increase and is predicted to reach 5700 × 106 t/year by 2030. In addition to expected oil price increases, further increases in greenhouse-gas (GHG) emissions are foreseen as a result of the extraction of oil, particularly from unconventional sources such as oil sands and oil shale. Decreasing our current oil dependence and reducing GHG emissions by shifting toward renewable raw materials are key goals of any biorefinery-related research.
The chemical and mechanical pulping of wood generates large amounts of side streams, of which Crude Turpentine (CT) is one of the most interesting. In fact, the estimated worldwide production of Turpentine is about 350000 t/year. Currently, in chemical pulping, the Crude Sulfate Turpentine (CST) is condensed from digester vapors. Although the sulfate method (the Kraft process) is the current predominant technology, some sulfite mills also still exist.Sulfite turpentine emerges when crude tall oil is skimmed from pulping liquor, neutralized with NaOH or lime, and subsequently distilled. In addition, minor amounts of high-purity Wood Turpentines are steamed directly from chopped tree trunks. Still, in mechanical pulping processes, terpenes are recovered from the mill by steam distillation. The estimated amount of turpentine produced depends highly on the tree species processed and reaches 0.3−1.0 and 2−16 kg/ton of pulp for the sulfite and sulfate processes, respectively. In most cases, roughly 75% of the native turpentine composition remains unchanged during the extraction. Still, thermomechanical pulping (TMP) processes typically allow for the recovery of 0.3 kg of turpentine per ton of pulp. Very often, these fractions are utilized onsite; they are commonly burned in recovery boilers to generate steam used by the mills, and also to produce electricity.
In addition, for a long time, turpentine has been utilized by the perfumery industry and as a solvent for dyes and varnishes. Furthermore, purified CST is potentially applicable as a 5−20% fuel blend because of its low volatility and advantageous volumetric net heat of combustion.
Herein, we review a series of important scientific advances made within the time frame of ca. 2000−2014 in the upgrading of α-Pinene, the predominant compound of crude turpentine, into value-added products through heterogeneous catalysis involving various different active species (such as metal functions or acid sites) on the catalyst surface. Moreover, various transformations of Limonene, Camphene, β-Pinene, and ρ-Cymene are also considered. Furthermore, several examples of successful applications utilizing crude turpentine are described. Evidently, several approaches taking advantage of heterogeneous catalysis have been developed and improved during recent decades involving materials that are highly selective toward the desired products, namely, catalysts with good reusability, low toxicity, simple handling, and easy disposal. In general, investigations concerning catalytic aspects, reaction parameters, and mechanistic pathways are also discussed and compared.
Turpentine is a collective term used for a mixture comprising numerous C10H16 monoterpene isomers. In general, bicyclic compounds such as 3-Carene, camphene, and α- and β-Pinenes, together with monocyclic Limonene, are the principal compounds of this raw material. The chemical composition of crude turpentine (CT) varies strongly with the wood species, biomass growth region, pulping process or mill, and even harvesting season (Table 1). For example, Kraft turpentine from the United States can contain more β-Pinene than α-Pinene. However, in turpentine originating from sulfite pulping, ρ-Cymene is typically the predominant compound.
Because of the use of sulfur-containing cooking chemicals upon pulping, the sulfur content in CT can reach 3 wt %, whereupon the three main species present are methanethiol, dimethyl sulfide (DMS), and dimethyldisulfide (DMDS). The organoleptic properties of the aforementioned malodorous organics complicate the further use and upgrading of CT, as well as the isolation and utilization of specific terpenes. Furthermore, sulfur is a well-known catalyst poison that efficiently compromises catalytic activity by means of poisoning and deterioration of the active sites, particularly in noble-metal catalysts. For further utilization of bicyclic monoterpenes, sulfur removal is required in such a way that the initial CT composition is maintained and formation of degradation products is minimized. Through the combination of various absorption, fractionation, hypochlorite, and metal treatment techniques, the sulfur content can be decreased.In addition, Knuuttila successfully used the hydrodesulfurization (HDS) of CST over mixed NiMo/γ-Al2O3 and NiW-NiO catalysts. Also, up to 80 wt % sulfur was eliminated upon HDS treatment with low substrate degradation in a study by Casbas et al., who used sodium doped Co and Mo catalysts. In contrast to that obtained from the sulfate and sulfite processes, TMP turpentine is sulfur-free and therefore of higher value. Direct catalytic upgrading of turpentine is, in general, complicated because of the high variety of its constituents. Evidently, in most pulping processes, pinene is the predominant turpentine compound, and thus, the appropriate catalyst selection and the desired process optimization should reflect this fact.
α-Pinene is a bicyclic terpene exhibiting a high reactivity that has been broadly studied in various reactions such as isomerization, dehydroisomerization, oxidation, hydrogenation, hydration, dimerization, and acetoxylation reactions.
Optical activity is an important aspect of monoterpene chemistry, and in some cases, it helps in elucidating the reaction mechanisms, providing explanations in terms of the interactions between the substrate and the catalyst and even in terms of tuning the catalyst stereoselectivity. Unfortunately, many authors do not specify the optical nature of the terpene reactant employed or the terpenoid obtained. The geometric (cis/trans) and stereoisomeric structures of monoterpenes, described in the current review, are depicted in Figure 1.
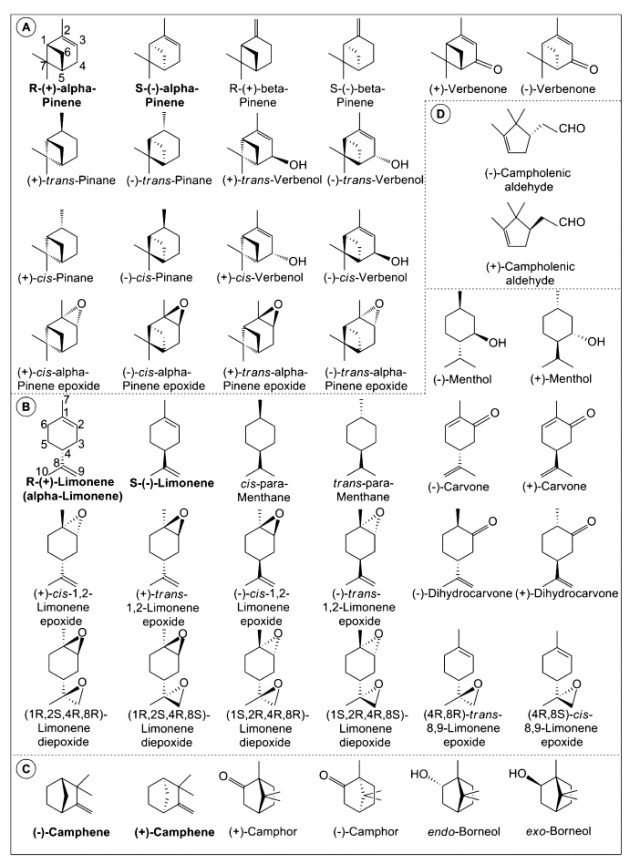
Figure 1. Absolute configurations of monocyclic and bicyclic monoterpenes and their derivatives described in the current review: (A) pinene and related, (B) limonene and related, (C) camphene and related, (D) campholenic aldehyde (five-membered ring). Adapted from refs 21−25, 30, and 31.
The optical activities of α- and β-Pinenes are represented by the chiral centers at C1 and C5 and defined by the spatial configuration of the sixth carbon atom. Other terpenoids with such bicyclic structures demonstrate similar stereoactivities (Figure 1).
In nature, two optical isomers exist for limonene: R-(+) and S-(−), with orange and lemon odors, respectively. In this molecule, the chiral center is situated at the fourth carbon atom of the limonene ring skeleton. Also, monocyclic terpene derivatives with related isomers demonstrate similar optical activities. However, an additional chiral center could also exist at the first carbon atom of the monoterpenoid ring if it were hydrogenated or functionalized at that position (Figure 1). Of the two above-mentioned isomers, the R-(+) isomer is the more abundant and accessible. Currently, related studies as described in this review are restricted to R-(+)-limonene. By default, most studies have been performed with R-(+)-limonene as the more reactive substrate, although studies comparing the relative activities of R-(+)- and S-(−)-limonene substrates have also appeared. Furthermore, the formation of various optical isomers is schematically presented for all described reactions.
Learn full content of artical
© 2015 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany
1. Introduction
2. Turpentine Composition of Upgrading
3. α-Pinene Upgrading Techniques
3.1. Isomerization
3.2. Isomerization of “Oxygenated Terpenoids”
3.2.1 α-Pinene Epoxide
3.2.2 Limonene Epoxide
3.3. Dehydroisomerization
3.4. Oxidation and Epoxidation
3.4.1 Oxidation and Epoxidation of α-Pinene
3.4.2 Epoxidation of Limonene and Camphene
3.4.3 Oxidation of ρ-Cymene
3.5. Hydrogenation
3.6. Acetoxylation
3.7. Dimerization
4. Conclusions
Author Information
Corresponding Authors
Notes
Biographies
Acknowledgments
References