Buyer Guide, Feature Article, Company News
Brief Description of cis/trans-Pinane
Buyer Guide, Feature Article, Company News

Linalool, known for its pleasant aromatic characteristics, is widely popular in the F&F market and is one of the highly traded monoterpenoid fragrances. It is also an important raw material for the synthesis of vitamin A and vitamin E. Synthetic-grade Linalool is favored by the cosmetics and pharmaceutical industries compared to natural Linalool.
| Name of Country | %, Component | ||
| α-Pinene | β-Pinene | β-Phellandrene | |
| China | 90 | 4 | 4 |
| U.S.A | 50 to 60 | 3 to 30 | 1 to 15 |
| South Africa | 53 | 23 | |
| EU | 50 to 66 | 13 to 18 | |
| Source: Components of turpentine oil in different countries, Guoen, 20011 | |||
Over the years, there have been two main synthetic schemes for the production of synthetic Linalool: A) Using petrochemical raw materials and the other using turpentine raw materials. In the early petrochemical route, acetylene and acetone were reacted in the presence of alkaline catalysts to produce Methyl heptenone through a series of catalytic reactions. Methyl hepten could be alkynylated by acetylene to obtain an alkyne alcohol intermediate, which was then hydrogenated to yield Linalool. In the mid-term petrochemical route, Isobutene was condensed with formaldehyde under high pressure to form methyl heptenone, which prepared to obtain Dehydrolinalool, and then hydrogenated again to produce Linalool. Alternatively, Isoprene could be catalytically condensed with acetone to form methyl heptenone, which was finally converted to Linalool.
B) The early route of turpentine production involved the thermal cracking of β-Pinene at high temperatures to convert it into Myrcene, which was then hydrolyzed with hydrogen chloride to obtain Linalool. This route had a high yield and was relatively simple, making the largest-scale industrial method for synthesizing Linalool in the world. Another synthesis route using Pinane hydroperoxides emerged as a new alternative. In this scheme, pinene was used as the raw material and underwent catalytic hydrogenation to produce cis-Pinane (When cis and trans isomer are expressed simultaneously it is called 2-Pinane), which is more prone to oxidation reactions (Brose Thomas, 1992)2. The cis-Pinane was oxidized to form Pinane hydroperoxides, which were then reduced to cis-Pinanol and finally subjected to high-temperature cracking to obtain Linalool. The challenge in this scheme in controlling the pyrolysis conditions of cis/trans-Pinanol during the cracking reaction, which can affect the final by-products and yield. Structurally, Pinane hydroperoxides belong to the class of Tertiary alkyl hydroperoxides and exhibit stability in the presence of alkaline catalysts. Pinane hydroperoxides can be catalytically converted to Pinanol in an alkaline sodium sulfide solution or in the presence of alkaline catalysts.
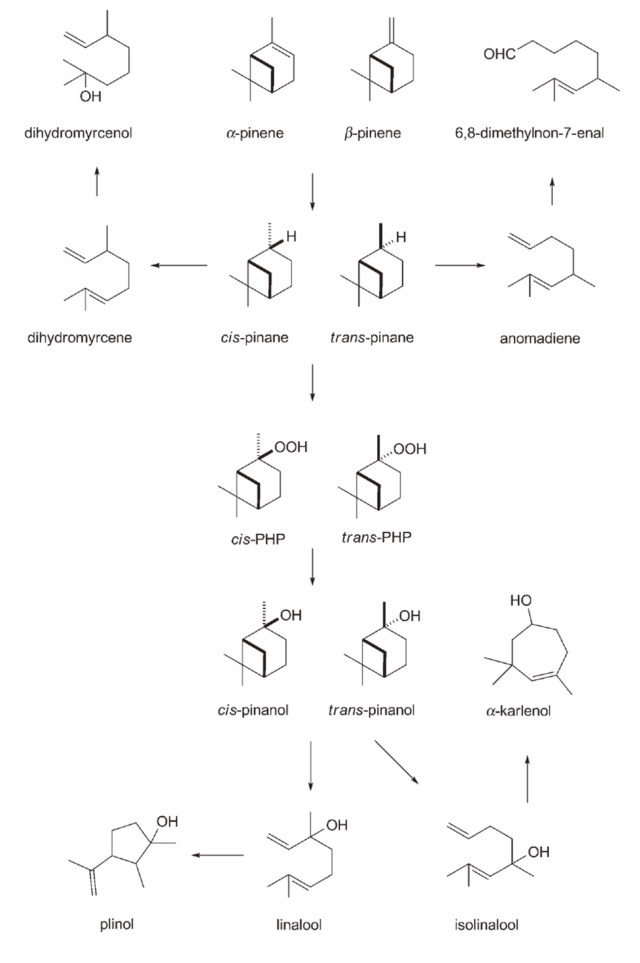
The synthetic schemes of pinenes. Source: Mark B.Erman, 2008
Saturated tertiary terpenols are a class of organic compounds classified as tertiary alcohols. These alcohols generally include cis-Pinanol, trans-Pinanol, Dihydroplinol, Tetrahydrolauryl alcohol, TetrahydroLinalool, Citronellol, Menthol, Opuntiol, and other valuable compounds. Natural saturated tertiary terpenols can be extracted and isolated from natural sources such as eucalyptus oil and other plant volatile oil. Due to their pleasant aroma, flavor, and potential therapeutic properties, they have wide applications in the fragrance, flavoring, and pharmaceutical industries. They can be prepared by hydrating certain monocyclic terpene compounds. In industry, saturated tertiary terpenols are extensively used in the production of plasticizers, surfactants, adhesives, resins, lubricants, and other chemicals. Additionally, they are employed in the preparation of fragrances and artificial milk.
Both cis- and trans-Pinane have corresponding Pinane hydroperoxides (cis-PHP and trans-PHP) which are used as polymer catalysts in devulcanization processes for recovered rubber. Researchers have demonstrated that chlorine oxidation reactions provide multiple schemes for pinonic derivatives (Mark B.Erman, 2008)3. cis-Pinane readily reacts with Hypochlorous acid (HOCl) to form chloro ketone substances. Under conditions of phase-transfer catalysis, chloro ketone substances react with primary alcohols to yield alkoxy ketones with floral and woody notes. By protecting the carboxyl group, ketal compounds with fresh, camphor-like, and minty aromas can be obtained. These ketals can further be transformed into ketones with a cedarwood-like scent and allyl alcohol with a sophisticated woody fragrance. Currently, cis-pinane is primarily used in the production of Linalool, which is then used synthesize Geraniol and subsequently Citronellol. On the other hand, the reaction of trans-Pinane with HOCI yields tricyclic ethers compounds, which have been proven to serve as intermediates for C(9)-functionalized fenchenes, cis-Bergamotenes, and the pheromone Grandisol of the Boll Weevil.