Industry News, Flavor & Fragrance Industry
An Aroma Chemical Profile – Benzaldehyde
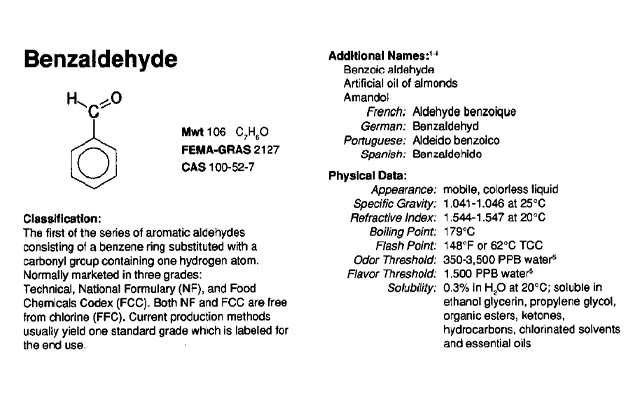
Industry News, Flavor & Fragrance Industry

Benzaldehde’s odor and taste are so unique snd basic dehyde. The odor has a pleasant, sweet, romatic note and the taste the same, with a slight, sweet, aromatic bite. We recall almonds, marzipan, peach pit meat (what youngster didn’t chew a peach pit nut?) and pistachio ice cream.
The industrial cliche for the product is “Bitter Oil of Almonds,” a name which has been misleadingly applied to the synthetic product since it emerged from tbe chemist’s flask before the year 1900. Small amounts of synthetic benzaldehyde produced from benzsd chloride first appeared on tbe market as natural oil of bitter almonds about 1890, Since the price ratio between the natural product and the synthetic is significant (currently 100 to 1), the Derfumer. flavorist and chemist have been constantly devising methods to differentiate the fraudulent product from the real thing.
The earliest detection methods6 utilized the precipitation of silver chloride by contacting the aqueous burned residues of benzafdehyde with silver nitrate reagent. Subsequent methods developed for use by both the US Pharmacopoeia and the Food Chemical Codex employ the visible green-blue color induced in a bunsen burner flame by a copper screen which has been dosed with the product. As industry moved away from the commercial production of benzaldehyde from benzal chloride to direct oxidation of toluene, which yields a product free of chlorine, fraud detection required advanced techniques. At first, carbon14isotope analysis was successful in detecting synthetic aldebyde being offered as natural. However, the price differential of $1.00/lb for synthetic versus $100.00/lb for natural benzaldehyde prompted tbe spiking of the synthetic nroduct with carbon-14 rich materiaf. thus cloakine its nat;re, Carbon-13/carbon-12 ratios (13C/12C) prove~ambiguous.7 The most recent successful method is deuterium/hydrogen isotope ratios.8 One might say that the crooks are encouraging the advancement of science.
The salient point of the above discussion underscores the true nature of oil of bitter almonds: it is afmost pure benzaldehyde, and pure synthetic henzaldehyde is nearly indistinguishable from the naturaf product. Moreover, during the period 1960 to 1990, almost no natural product was produced and all the “natural” being sold was synthetic, so the question of an organoleptic difference was a moot one.
As with many desirable aroma chemicals, there is no direct plentiful natural source for benzaldehyde. Currently, the sole noncontroversial commercial source of natural benzafdehydeg is the nut meat of apricots, peaches, prunes (plumbs) and bitter almonds. The ddehyde is tied up as a glucOside (amygdalin) and Can be released by enzymatic hydrolysis treatment of the meat. The yield based on dry nut meat is about 0.75% by weight. Current production is based on the by-product nuts from fruit processing of peaches, apricots and prunes (plumbs). It is estimated that about 20,000 kg of natural oil of bitter almonds are produced annuafly from these sources and that this production is of recent development (since 1990). Table I presents an estimate of the sources avaiiable should all the nut meat from dlthefmit grown reutilized. One should note that all the nuts would not be avaifable due to retail consumption of the fresh fruit.
| Table I. Potentiai sources of bitter aimond oil Worid total 1994 | |
| apricots | 1,550,000 kg |
| peaches | 7,309,000 kg |
| prunes (plumbs) | 5,210,000 kg |
| Total | 14,069,000 kg |
The potential available volume of naturaf benzaidehyde from these sources (Table 1) is estimated to be more than 7,000 Mtons, and can satisfy the current world demand for its use in flavors (Table II).
| Table II. Worid fiavor usage of benzaldehyde in 1994 by geographical region | |
| North America | 230,000 kg |
| Europe | 330,000 kg |
| Other | 245,000 kg |
| Total usage | 805,000 kg |
As benzaldehyde usage in flavors is probably growing at a 6% per annum rate, and will possibly continue as such over the next decade, even the potential supplies from the currently used sources will be adequate into the next century.
The issue of potential new plant sources was addressed by Lawrence10 and is presented in Table III.
| Table III. Potentlai new commercial source of natural benzaidahydahyde | |||
| Species | Oil yleld (%) | Amount (%) | Plant part |
| Eucalyptus yarrensis Maiden et Cambage |
0.1 | 90 | L/T |
| Prunus laurccerasus L. | <0.1 | 82 | F/L |
| Ziefia compasta Sm. | 0.1 | 90 | L/T |
| Z. cytisoides sens,strict | 0.4 | 55 | L/T |
| Z. laevigata var. fraseri | 1.1 | 80 | L/T |
| Z. smithii Andrews | 0.5 | 50 | L/T |
| L/T = leaves and twigs; F/L =fruit and leaves | |||
The supply of “natural” benzafdehyde obtained from natural cinnamic aldehyde ex-cassia oil is presently a dominant market source and is sold as natural benzaldehyde cassia distillate or roasted cassia oil. A purely thermaf cracking of cinnamic aldehyde to benzaidehyde would result in low yields and poor organoleptic quality if no cataiysts were employed. The use ofinorganic bases as a catalyst would place the “natural” label in question, Thus this source of “natural” benzaldehyde is questionable at least on a theoretic chemical basis.
Benzafdehyde is responsible for the taste and odor of almonds, and this association has overshadowed the fact that benzaldehyde is found as a glycoside in the pit meat of most of the fruits of the Prunus family (i.e., apricots, cherries, nectarines, peaches and plums). Thus, the crude benztddehyde-containing oils isoiated from these “nuts” are commonly referred to as oil of bitter almonds, irrespective of the actual source, Identifying the chemical responsible for this odor proved a “hard nut to crack” for the pioneers in organic chemistry. Investigators as early as 1803 were aware that tbe extractable organics contained a materiaf with the benzaldehyde odor, but isolation attempts to capture this material led to the discovery of only benzoic acid, due to the facile oxidation of the benzaldehyde during the work-up procedures employed.
Benzaldehyde oxidizes so easily that if a stream of air is bubbled through a container of it, crystals of benzoic acid will appear as a precipitate within 30 minutes, One laboratory wastebasket fire witnessed by this author was due to the spontaneous ignition of paper towels used to clean up a lab benzaldehyde spill. The fire was the result of benzddehyde’s rapid oxidation and its exothermictemperature rise to the flash point.
Early investigations also revealed the presence of hydrogen cyanide (prussic acid) in these nuts and the recognition of its toxicity.
It was the search for the nature of the benzene radical that spurred investigators to explore this area, and in 1832 Wohler and Liebig, in a mere two-month period, solved the problem with the discovery of benzaldehyde. The discovery was published in landmark paper which elucidated the structure of the benzoyl radical.11
In 1863, Cahours synthesized benzaldehyde via the hydrolysis of benzal chloride, thus unlocking the chemistry for future commercial production. However, the first significant commercial production of benzddehyde from benzaldehyde from benzal chloride was not realized until about 1900, as it was dependent on the commercial availability of benzyl chloride. Benzaf chloride production via the chlorination of toluene was dependent on the following developments:
Thus, after the discovery of the chemical process for the production of benzaldebyde, it took almost 40 years to develop the supporting technology that would allow significant commercial availability.
As in the case of benzyl alcohol, the synthetic dye industty’s requirement for benzyl chloride was the impetus for the commercialization of the technology The production of benzaldehyde was a result of by-product benzal chloride formed in the chlorination of toluene. The first sythetic benzaldehyde seems to have been produced by Schimmel in Leipzig (Germany) and sold as “Bitter Almond Oil” (Bittermandelol).
Between 1900 and 1910, essential oil houses in Germany and France soon learned to produce synthetic bitter almond oil and market it as natural oil, The purchasers soon learned of the hoax and developed methods of detecting the chlorine residues found in the synthetic product that were not in the natural oil. By 1925, the industry had developed residual organic chloride tests based upon silver nitrate reagents6 and the copper wire test used today by the Food Chwnical Codex for FFC grade, The ruse of selling spthetic benzaldehyde as natural continued until the last few years, as the supply of natural was limited or nonexistent and the price differential was great, The FCC test will detect chlorine residue to about .40 ppm. This level was assumed safe until benzd chloride was found to be a carcinogen and subsequently appeared on the proscription list of California’s Proposition 65.
Benzafdehyde forms an azeotrope with benzyl chloride (177.9”C) which is so close to the benzaldehyde boiling point (178.9”C) that its separation is difficult. Thus, commercial benzaldehyde produced from chlorinated toluene feed stocks invariably have small amounts of benzyl chloride residues. In the 1950s, Dow Chemical developed a liquid phase, air oxidation route for the production of benzoic acid, with the purpose of producing phenol from the acid. This process produces a benzafdehyde by-product which is totafly free of chlorine. The reaction was commercialized by Dow and the production facilities it built were later sold to Kalama Chemical and Chatterton Petrochemical. In Europe, Rhone Poulenc and DSM also began production of benzoic acid and benzaldehyde via similar routes. The availability of chlorine-free benzafdehyde spelled the death of the product made by the chlorination route, although production of benzafdehyde from benzaf chloride did not cease until 1993.
Today, no major global manufacturer produces benzddehyde from chlorinated feed stock,
Chlorination of toluene: Tbe major synthetic process employed from 1900 to about 1970 for the manufacture of benzaldebyde [3] was the chlorination of toluene followed by the hydrolysis of the by-product benzaf chloride [2] (Figure 1).
The benzaldehyde [3] thus produced was contaminated with smafl amounts of organic chlorides, which, if kept below .40 ppm, were never considered apmblem for me in flavors m fragrances until benzal chloride [2] was found to be a carcinogen, Production of benzafdehyde via this process started to decline in 1960, and ceased in 1993.
Direct oxidation of toluene: In the 1950s, Dow Chemical developed a direct air oxidation of toluene in the liquid phase using cobalt catafysts (Figure 2), The process was designed to provide benzoic acid to be used as a feed stock to produce phenol. The initial step of toluene oxidation produces about 4% by-product benzafdehyde which is marketed at $1.00 -1,40/fb versus the selling price of $0,31/lb for phenol; in other words, the by-product is worth more than the product.
Synthetic benzakfeltyde: It is estimated that world usage of benzafdehyde in 1994 was approximately 13,000 Mtons of which about 6,400 Mtons were consumed directly or indirectly by the world flavor and fragrance industry The major world producers of synthetic benmfdehyde are presented in Table IV.
| Table IV. Producers of synthetic benzeldehyde | ||
| Firm | Capacity | Process |
| DSM | 8,000 Mtons | air oxidation |
| Kalama | 3,600 Mtons | air oxidation |
| Others | 2,000 Mtons | mainl air oxidation |
| Total | 13,600 Mtons | |
Natural benzaldehyde The natmzd benzaldehyde market is currently at about 100 Mtons/y worldwide, and growing at about 5% per year. The major volume sold, about 80 Mtons, is natural benzafdehyde ex cassia oil, The remaining 20 Mtons of product is natural Bitter Oil of Almonds obtained from peach and apricot pit meat. The questionable natural label of benzaldehyde ex cassia oil has been conveniently ignored by the flavor industry as the two products vary so widely in price. For example, natural Bitter Oil of Almonds is selling in the $130- 180/lb range while benzaldehyde ex cassia oil is priced at $50-55/fb,
World consumption of benzaldehyde for all uses in 1994 is estimated at 13,000 Mtons. Usage in the flavor and fragrance industry is broken out in Table VI.
| Table VI. Eetimeted benzeldehyde uesge in the flsvor snd frsgrsnce induetry in Mfone 1994 | ||||
| North America | EEC | Other | Total | |
| flavors | 215 | 380 | 255 | 850 |
| fragrances | 50 | 100 | 130 | 280 |
| aroma chemical intermediate | 1,000 | 2,300 | 2,000 | 5,300 |
| Total | 1,265 | 2,780 | 2,385 | 6,430 |
The remaining 6,570 Mtons of benzaldehyde consumed in 1994 was used as a feed stock for benzyl alcohol, benzyl amine, benzylidene acetone, benzyl acetone, dibenzyl amine, pesticides and various other low-volume products. The direct flavor and fragrance industry usage of benmddehyde is only about 8’%of world production, the indirect usage (as amyl cinnamic aldehyde and cinnamic aldehyde) adds another 41% to world consumption. Thus, our industry is responsible for about 49% of the worlds usage of this product.
Benzaldehyde is currently selling in the $0.95- l.20/lb range, depending on grade. It is produced as a by-product by phenol manufacturers, and with phenol currently selling at about $0.3 l/lb, benzaldehyde is clearly a premiumpriced product. The choice of whether to recycle the benzaldehyde to make phenol or sell it “as is” is rather clear. Part of the current tightness in the enzyl alcohol supply is because it sells at $1.10/lb and is largely produced from the benzaldehyde by-product. Thus, manufacturers have chosen to sell benzaldehyde rather than reduce it to the alcohol.
Benzafdehyde is currently listed under the Harmonized Tariff No. 29121.21.00008 and bears a 9% duty Imports to the US have grown recently (Table VH) and now represent about 16% of US current demand.
Numerous materials have odor-taste profiles near benzaldehyde, but few can be used because of product safety considerations. The most successful substitute is methyl benzaldehyde (toluafdehyde [4]) (Figure 3).
Approximately 45,000 kg of toluddehyde are used in flavors worldwide; about 16,000 kg of that volume are used in the US. The major producers of the p-toluafdehyde are BASF and Mitsubishi Gas Chemical Co. Givaudan-Roure offers a mixture of ortho, meta and para isomers.
Benzafdehyde’s analogues are interesting in their own right and afso to illustrate organoleptic shifts (Figure 4).
Small bulky groups, substituted mainly in the para position of the ring of the afdehyde, show benzaldehyde type odors([5]-[8]). However, larger aflgd groupings or destruction of the aromaticity of the ring result in a shift to the green-herbaceous odor class ([9]-[16]). If more polar substituents are selected, afloral cla.ssis created ([17]-[22]). If the number of polar groupings is increased to excess, a vanillic impression results ([27]-[30]). If the substitutions of increased bulk area mixture of polar and nonpolar (afkyl) groups, the odor shifts to the musk cla.ss ([23] -[26]).
If derivatives include alcohols or condensation products with other afdehydes (such as the cinnamates), then the benzaldehyde derivatives used in the flavor and fragrance industry are mainly its acetals (Figure 5), which impart a benzaldehyde note for formulations in which benzaldehyde is unstable.
Address corrsspondsnce to George S. Clark, Commodity Services International, lnc, PO Box 1876, Easton, MD2i601 USA.
1. The Givaudan Index, 2nd sd., New York: GivaudanDslawanna, Inc. (1961) p 65
2. Thekferck/ndex, No 1054, 10thsd, Rahway, New Jersey: Merck &Co (19S3)
3. The National Formtdary, Rockville, MarJand: United States Pharmacopeial Convention, Inc, NF18(1995) P 2218
4. Food Chemical Codex, 3rd ed, Washington, OC: National Academy Press (1961 ) p 358
5. JC Leffingwell and D Letingwell, Perf& F/av 16(1 ) 13(1991)
6. EJ Parry, Parry’s Cyc/opaedia of Perfumery, Philadelphia: P Blakiston’s Son (1925) PP 60-81
7. JA Buchel, Flavoring with essential oils, incommercial papers from the Proceedings 1Ith International Congress of Essential Oifs, Fragrances and Flavor2, Novi2-16, 1989
8. L Trinnaman et al, Proceedings of the 12th International Congress of Flavors, Fragrances and Essential Oi/s, Vienna, Austria, 0ct4-8, 1992, Post Congress volums, pp 135-140
9. N Shaath and B 8enveniste, Natural oil of bitter almond, Perf & F/av16 (6) 17-24 (1991)
10. B Lawrence, Peti & F/av 17(5)20 (1992)
11. F Wohlerand JV Liebig, Untersuchungon uebsrdas Radikal der Benzoiesaeure, Ann 16321,Hi,pp 249-282