R&D
Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1beta, IL-6 and IL-10 on human macrophages

R&D

Objective Tea tree oil (TTO) is an essential oil with antiinflammatory properties, steam distilled from the plant Melaleuca alternifolia. We investigated the immunomodulatory properties of TTO and its components (terpinen-4-ol and alpha-terpineol) using lipopolysaccharide (LPS)-stimulated macrophages.
Methods The ability of TTO, terpinen-4-ol and alphaterpineol to modulate the macrophage response to bacterial LPS stimulation was assessed by ELISA for tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-10 cytokine production and by western blotting for the activation of nuclear factor kappa B (NF-κB) and p38 mitogen-activated protein kinase (MAPK) signaling, which are associated with the expression of pro-inflammatory cytokines. We used a human monocytic cell line (U937) differentiated into macrophages.
Results LPS induced the production of all cytokines, and TTO and its components significantly reduced the production of IL-1β, IL-6 and IL-10. The production of TNF-α was not affected by either TTO or its major components. The modulation of cytokine production was not mediated by changes in NF-κB or p38 MAPK activation.
Conclusion TTO, terpinen-4-ol and alpha-terpineol can suppress the production of inflammatory mediators in LPSstimulated human macrophages; this inhibition was mediated by interfering with the NF-κB, p38 or ERK MAPK pathways.
Keywords Tea tree oil, Macrophages, Cytokines, Inflammation
Melaleuca alternifolia of the botanical family Myrtaceai is a plant native to Australia also known as tea tree. The steam distillation of its leaves produces an oil known as tea tree oil (TTO). TTO is a mixture of approximately 100 compounds, including the monoterpenes terpinen-4-ol (at least 30 % of the oil), 1,8-cineole and terpinolene [1–4]. TTO became known worldwide in the eighteenth century, and possesses antimicrobial [5–8], anticancer [9] and antiinflammatory properties [10].
Randomized controlled clinical trials have demonstrated the efficacy of TTO in reducing inflamed acne lesions with topical use [11]. In patients with herpes labialis, the reepithelialization of this lesion is faster with the use of topical TTO [12]. In dental applications, TTO significantly reduces the gingival index and papillary bleeding index score [13]. Terpinen-4-ol has shown inhibitory effects on the inflammatory response, mainly due to a reduction in vasodilation and plasma extravasation. Regarding alphaterpineol, anesthetic properties have been found [14].
The anti-inflammatory mechanism of TTO occurs via the suppression of superoxide production and inhibition of inflammatory mediators such as TNF-α, IL-8, IL-1β and prostaglandin E2 (PGE2) in human monocytes. The oil interferes with cytokine secretion by lymphocytes and monocytes, and also decreases the production of IL-2 and increases IL-4 and IL-10 expression [15]. It reduces the production of reactive oxygen species and cyclooxygenase activity, but the migratory capacity of polymorphonuclear leukocytes (PMNs) is unchanged in the presence of TTO [15]. The anti-inflammatory activity of TTO in terms of inhibiting neutrophil adhesion is weak at concentrations of 0.016–0.033 % [1]. Since the available evidence indicates that TTO does not affect neutrophils, it has been suggested that the mechanism of action of the oil is selective regulation of the function of monocytes during inflammation[1].
The most abundant component of TTO, the terpinen-4-ol, is able to repress (by 40–50 %) the secretion of cytokines such as IL-1β, TNF-α, IL-10 and PGE2 by LPSstimulated human monocytes [16]. However, the mechanism of action of alpha-terpineol does not interfere with cytokine production, but rather suppresses the production of superoxide [17].
The activation of Toll-like receptors (TLR) by their interacting ligands triggers a series of events in an attempt to eliminate the pathogen through the release of a number of cytokines, antimicrobial peptides and chemokines [18]. NF-κB signaling is especially relevant for the expression of inflammatory cytokines upon TLR activation [19]. Therefore, strategies aiming at inhibiting NF-κB activation have the potential to modulate the development of inflammatory disease by interfering with the cytokine network [18, 19]. Despite some knowledge on the modulation cytokines by TTO and its components, no studies have assessed the activation of NF-κB and MAPK under these conditions.
TTO has a demonstrated ability to suppress the in vitro production of inflammatory cytokines, suggesting its potential as a therapeutic agent for inflammatory diseases, such as periodontal disease, via modulation of the host response. Thus, the objective of this study was to evaluate the ability of TTO and some of its components to modulate the production of LPS-induced inflammatory cytokines by macrophages in vitro. Moreover, we assessed if this modulation is dependent on the activation of TLR2/TLR4 and involves NF-κB or MAPK signaling.
The composition of TTO (Sigma-Aldrich St. Louis, MO, USA) may vary according to the extraction protocol and geographic region from where the plant was obtained. To verify that the TTO was within the ISO standards, a gas chromatography was performed using a Shimadzu GC 17A gas chromatograph with a nitrogen–phosphorus detector. 1 μL of TTO was dissolved in a 1 ll ethanol and injected into a capillary column HP35 (25 mm 9 0.25 mm 9 0.33 μm) and carrier gas and with flow rate was 0.8 mL/min. The temperature of the injector and detector was 250°C. The temperature program was: initial temperature 80°C for 2 min, the heating ramp 10°C/min up to 250°C and kept for the final 10 min [20, 21].
The concentrations were determined from the time of fractionation. Gas chromatography showed that the TTO was according to International Organization for Standardization standard no. 4730 (IOS 4730) and an evaluation of the relative concentrations of the components showed that terpinen-4-ol was 47.66 and 5.13 % alpha-terpineol. The components terpinen-4-ol and alpha-terpineol (Sigma-Aldrich St. Louis, MO, USA) were obtained individually.
U937 cells, a monoblastic leukemia cell line were maintained in RPMI-1640 culture medium (GIBCO Life Technologies, Carlsbad, CA, USA) with 10 % heat-inactivated fetal bovine serum (FBS) (GIBCO Life Technologies, Carlsbad, CA, USA) and 1 % penicillin–streptomycin (GIBCO Life Technologies), in a humidified atmosphere of 5 % CO2 at 37 C. Cells were seeded at a concentration of 25 9 104 cells/mL in 182 cm2 cell culture flasks.
Differentiation of monocytes into macrophagic adherent cells
Differentiation was induced using 40 ng/mL PMA (phorbol 12-myristate 13-acetate; Sigma-Aldrich) [22]. U937 cells were dispensed into well plates (1.0 9 106 cells/mL) and incubated with PMA for 24 h at 37 C in a 5 % CO2-humidified atmosphere. After PMA treatment, the culture medium was removed and the wells were washed three times with phosphate buffered saline (PBS) to remove remaining PMA and non-adherent cells. Differentiated cells were further maintained in culture (RPMI with 10 % fetal bovine serum and 1 % antibiotics), for 24 h (recovery phase).
Macrophages were stimulated with ultrapure LPS from Porphyromonas gingivalis at 1 lg/mL (InvivoGen, San Diego, CA, USA). This LPS has been shown to activate TLR2 and TLR4 [22]. We also stimulated cells with ultrapure LPS from Escherichia coli, also at 1 lg/mL (InvivoGen), considered to be a specific activator of TLR4[23].
The following stock solutions of TTO were prepared: TTO 0.25 % (v:v or 2.24 mg/mL); terpinen-4-ol 0.238 % (2.22 mg/mL); alpha-terpineol 0.0064 % (0.0595 mg/mL) with RPMI-1640, 1 % inactivated fetal bovine serum without penicillin–streptomycin and 0.4 % DMSO (dimethyl sulfoxide; Sigma-Aldrich) as a solubilizing agent [17].
These stock solutions were further diluted to the following final concentrations in culture medium with 1 % FBS in the wells containing macrophages: TTO 0.125 % (1.125 mg/mL), 0.062 % (0.556 mg/mL), 0.031 % (0.278 mg/mL), 0.015 % (0.134 mg/mL), 0.008 % (0.0718 mg/mL) and 0.004 % (0.0359 mg/mL); terpinen-4-ol 0.119 % (1.111 mg/mL), 0.059 % (0.5510 mg/mL), 0.029 % (0.2708 mg/mL), 0.014 % (0.1307 mg/mL) and 0.0073 % (0.0681 mg/mL) and alpha-terpineol 0.0031 % (0.0288 mg/mL), 0.0015 % (0.0139 mg/mL) and 0.0007 % (0.0065 mg/mL) used in the wells containing the macrophages (protocol previously) and different concentrations of oil. After 24 h, the medium containing TTO or its components was removed and each well was washed three times with PBS and fresh RPMI-1640 medium without FBS and supplemented with 0.5 mg/ml of MTT salt [3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide; Sigma-Aldrich] and incubated for 4 h at 37°C. The MTT solution was replaced with 300 μL of acidified isopropanol (5 mM HCl) and the absorbance was measured at 550 nm (Bio-Rad, model 3550-UV microplate reader, Hercules, CA, USA). The blank solution was discounted for each oil to avoid any interference.
Macrophages were washed twice with PBS and the medium was replaced with medium containing the highest and lowest non-cytotoxic concentrations of oil and its components, previously determined by the cytotoxicity assay. The preparation of the solutions followed previously described protocol. After the addition of oil, the cells were incubated for 2 h [24], and after this period, the cells were stimulated for 24 h with either P. gingivalis or E. coli ultrapure LPS (37°C, 5 % CO2) according to the following experimental groups: (1) no stimulation (negative control); (2) 0.4 % DMSO (negative control, vehicle); (3) same concentration of the oils without LPS stimulation (negative control, treatment); (4) only LPS stimulation with either LPS from either P. gingivalis or E. coli (positive control, stimulus); (5) pre-treatment with the oils followed by stimulation with either LPS (experimental groups, treatment).
Macrophages in 60 cm2 plates were treated with 0.015 % TTO, terpinen-4-ol 0.059 and 0.0064 % alpha-terpineol in RPMI-1640 containing 1 % fetal bovine serum and 0.4 % DMSO. After incubation for 2 h, some groups received only oils and other groups received oils and were stimulated with P. gingivalis or E. coli LPS kept for 10 min to assess the activation of signaling pathways of interest. Control groups were negative and positive macrophages without stimulation and with each of the two LPS preparations, respectively.
Total proteins were extracted using a protease and phosphatase inhibitor cocktail (Complete Mini EDTA-free and PhoStop, Roche Applied Science, Mannheim, Germany; M-Per, Thermo Scientific Rockford, IL USA) according to the manufacturer’s instructions.
The proteins were quantified (20 lg) by the Bradford method [25] and subjected to heat denaturation, with the addition of sample buffer containing 0.1 M dithiothreitol (DTT). The proteins were subjected to electrophoresis (SDS-PAGE) on discontinuous polyacrylamide gels (10 % Mini Protean TGX, Bio-Rad Laboratories) at a constant voltage of 100 V for 90 min, followed by electro-transfer to nitrocellulose membranes (porosity 0.2 lm) for 1 h at 300 mA in transfer buffer containing methanol.
Blocking was performed with 5 % skim milk in Tris–NaCl (TBS) for 2 h at room temperature. The membranes were incubated for 16–18 h (overnight) at 4°C under gentle agitation with primary polyclonal antibodies reactive against the phosphorylated forms of p65 (NF-κB), p44-42 (extracellular signal-regulated kinases, ERK1/2) and p38 MAPK (all from Cell Signaling, Danvers, MA, USA). Verification of equal loading was assessed by the expression of GAPDH (also from Cell Signaling, Danvers, MA, USA). Detection of the primary antibodies was done using species-specific secondary antibodies conjugated to the enzyme horseradish peroxidase (HRP) with 1 h of incubation under gentle agitation at room temperature. Detection of primary/secondary antibody complexes was performed using a chemiluminescence enzymatic substrate (Supersignal West Pico, Pierce Biotechnology) on a digital documentation system (Chemi-Doc XR, Bio-Rad).
Role of NF-κB and MAPK signaling on modulation of cytokine production by TTO and its components
We used specific biochemical inhibitors for NF-κB (Bay11-7082, 10 lM), ERK1/2 (PD98059, 10 μM) and p38 MAPK (SB203580, 10 μM) (all from InvivoGen). All inhibitors were added prior to (40 min) stimulation with either P. gingivalis or E. coli LPS, and stimulation was carried out for 24 h.
The experiments were performed with replicates and repeated independently three times. Subsequently, the supernatant was collected, centrifuged at 13,000 rpm at 4°C, aliquoted and stored at -80°C. The total protein concentration in the samples was measured by the Bradford method [25] and used for normalization of the cytokine concentration.
The supernatant was analyzed using the Bio-Plex Cytokine Assay according to the manufacturer’s instructions (Bio-Rad Laboratories). Briefly, samples were incubated with beads at room temperature for 30 min, washed, incubated with biotinylated anti-cytokine antibodies for 30 min and subsequently for 10 min with streptavidin–phycoerythrin. The concentrations of cytokines were measured using a Luminex 96-well plate reader (Bio-100-PlexTM Multiplex-Suspension Array Reader, Bio-Rad Laboratories) and analyzed using Bio-PlexTM Manager Software 4.1.1. Recombinant human cytokines were used as standards.
Before analysis, we tested data normality by the Shapiro–Wilk test, and significance was assigned a value of p\0.05. In the cytotoxicity assay, data were analyzed relative to the absorbance values of the negative controls and expressed as percentage change (negative control= 100 %). For comparisons between experimental groups, we used ANOVA followed by the Tukey post hoc test. To assess the modulation of cytokine production, we used t tests for independent samples, for pairwise comparisons between the LPS stimulation alone and LPS stimulation in the presence of TTO or its major components both with and without biochemical inhibitors of signaling. All analyses were performed using GraphPad Prism 5.
Figure 1 shows cell viability at various concentrations of TTO. TTO at 0.25, 0.125, 0.062 and 0.031 % was cytotoxic to macrophages. However, at concentrations up to 0.015 %, there was no cytotoxicity, so we used 0.015 % as the high and 0.004 % as the low concentration. DMSO at 0.4 % did not affect cell viability. Terpinen-4-ol was cytotoxic at 0.238 and 0.119 %, so we used 0.059 and 0.0073 % as the high and low concentrations in subsequent experiments. For alpha-terpineol, since none of the tested concentrations reduced viability by 50 % or more in comparison with the negative control, we used 0.0064 and 0.0007 % as the high and low concentrations.
TTO, terpinen-4-ol and alpha-terpineol modulate the production of TLR4- and TLR2/TLR4-induced IL-1β, IL-6, IL-10 but not TNF-α
To investigate the effect of the oils on cytokines produced by macrophages upon TLR4 and TLR2/TLR4 activation, we added a treatment control group, in which cells were treated with the same concentration of TTO or its major components in the absence of LPS stimulation. This group allowed us to verify that treatment with TTO or its major components had no effect on cytokine production in unstimulated macrophages (data not shown). Also, treating the cells with the same volume of the DMSO-containing diluent (negative control vehicle) did not affect the production of cytokines (data not shown), indicating that the solvent used for diluting the oils was innocuous at the concentration used.

Figure 2a, b illustrates the effect of LPS from E. coli and P. gingivalis with the high and low concentrations of TTO, terpinen-4-ol and alpha-terpineol on the secretion of TNF-α. Both TLR2/TLR4 (P. gingivalis LPS) and TLR4 (E. coli LPS) effectively induced the production of IL-1β (Fig. 2c, d), IL-6 (Fig. 2e, f), IL-10 (Fig. 2g, h) and TNF-α (Fig. 2a, b), with higher potency for the stimulation of TLR4 by E. coli LPS, except for IL-10 (Fig. 2g, h), which was induced by P. gingivalis and E. coli LPSs with the same potency.
Overall, treatment with TTO or alpha-terpineol had no or minor effects on the production of IL-1β in macrophages after TLR4 or TLR2/TLR4 stimulation, with terpinen-4-ol resulting in a more consistent inhibition of IL-1β (Fig. 2c, d). The effects on IL-6 (Fig. 2e) production were similar to those on IL-1β (Fig. 2c), except for a significant inhibition of IL-6 (Fig. 2e) with the high concentration of alpha-terpineol. Intriguingly, anti-inflammatory IL-10 (Fig. 2g, h) was most potently inhibited by TTO and its major components at nearly all the concentrations tested. Even though E. coli LPS (TLR4 agonist) (Fig. 2g) was a more potent inducer of cytokine production by macrophages than P.gingivalis LPS (TLR2/TLR4 agonist) (Fig. 2h), the effects of the oils on cytokine production were similar for both LPS preparations, except for a significant inhibitory activity of the low concentration of alpha-terpineol on P.gingivalis LPS-induced production of IL-1 β and IL-6 (Fig. 2d, f).

Importantly, the oils alone (in the absence of LPS stimulation) did not modulate TNF-α, IL-1β or IL-6 production. However, terpinen-4-ol at 0.059 % and alphaterpineol at 0.0064 % reduced IL-10 production (data not shown).
Next, we verified that the production of these target cytokines in our in vitro system was mainly dependent upon NF-κB and p38 MAPK activation (ERK MAPK activity was also found to be important, albeit less critical) by pre-treating macrophages with specific biochemical inhibitors before incubation with TLR4 (E. coli LPS) or TLR2/4 (P. gingivalis LPS) agonists (Fig. 3).

Inhibition of TLR4 and TLR2/4-induced cytokine production by TTO, terpinen-4-ol and alpha-terpineol is not dependent on NF-κB, p38 and EKR MAPK
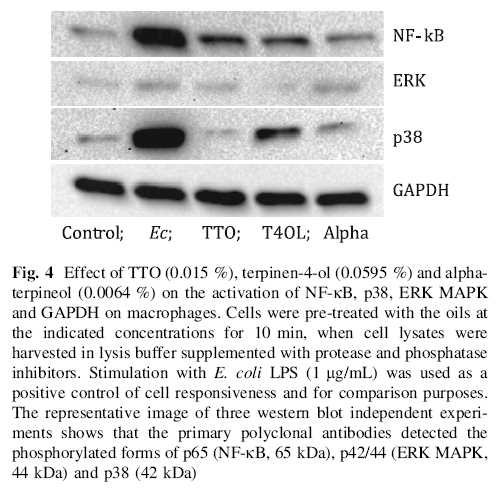
When used alone, TTO, terpinen-4-ol and alpha-terpineol did not induce a significant and consistent activation of NF-κB, p38 or ERK MAPK. The responsiveness of the macrophages was verified by using E. coli LPS as a positive control stimulus (Fig. 4).
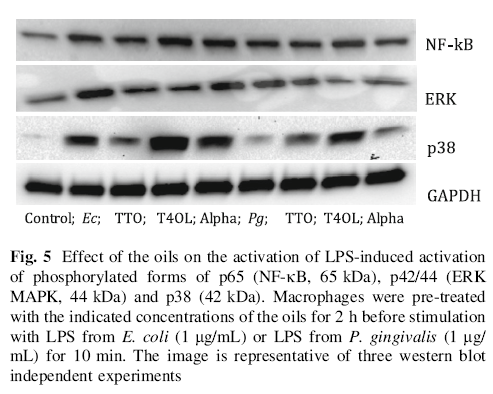
In Fig. 5 both E. coli and P. gingivalis LPS induced activation of NF-κB, p38 and ERK MAPKinases and E. coli LPS was noticeably more potent in this activation. We then used the oils as a pre-treatment before stimulation with LPS from E. coli or P. gingivalis for 10 min (Fig. 5); TTO decreased E. coli LPS-induced activation, but not P.gingivalis-induced activation, of all three pathways, especially of p38 MAPK. T4OL only reduced ERK activation induced by both E. coli and P. gingivalis LPS, and Alpha reduced P.gingivalis-induced, but not E.coli LPS-induced, activation of NF-kB and ERK. Interestingly, treatment with all the oils, except for TTO/E.coli LPS, increased activation of p38 MAPK in comparison to stimulation with either LPS alone.
In this study, we investigated the potential of TTO, terpinen-4-ol and alpha-terpineol to modulate the production of cytokines characteristic of the innate immune response, using an in vitro system with ultrapure LPS from different bacterial species as activators of TLR4 or TLR2/4 in human macrophages. We found that TTO, in particular its component terpinen-4-ol, could significantly inhibit cytokine production associated with the innate immune response.
The oil composition of plant extracts may vary according to the extraction method and geographic region where the plant was obtained. Therefore, to be referred to as TTO, ISO 4730 has established standards for known concentrations of the components [1]. Gas chromatography confirmed the framing ISO patterns of TTO, expressed as the relative concentrations of terpinen-4-ol and alpha-terpineol.
We initially assessed the cytotoxicity of the oil and its constituents, as the modulation of cytokine production could be associated with the induction of cell death [10, 26]. We arbitrarily considered as non-cytotoxic the highest concentration of TTO and its constituents associated with less than a 50 % decrease on cell viability; however, with
this approach, we cannot absolutely rule out the influence of a moderately cytotoxic effect at the highest concentration. However, we also used a lower concentration to assess a possible dose-dependent effect and to allow for a reduction in the possible bias associated with cytotoxicity (Fig. 1).
TNF-α plays a central role in the inflammatory response. Production of TNF-α induced by activation of TLR4 or TLR2/TLR4 was not modulated by TTO or its components (Fig. 2a, b), which contradicts the report of Hart et al. [10] showing that terpinen-4-ol decreased the levels of TNF-α. The difference between this work and the study by Hart
et al. [10] may be due to the cell type and duration of exposure to the oil, as these authors used monocytes from the peripheral blood of human donors and the oils were incubated with the cells for 40 h in the presence of E.coli LPS (500 ng/mL).
In contrast to TNF-α, IL-1β production was inhibited by TTO and its components, but this effect was not dosedependent. TTO was effective only upon TLR4 whereas alpha-terpineol was effective only after TLR2/4 activation. Terpinen-4-ol significantly inhibited IL-1β production after both TLR4 and TLR2/4 stimulation (Fig. 2c, d).
TTO also inhibited the production of IL-6 after the activation of TLR4, but not after TLR2/4 activation (Fig. 2e, f), suggesting that signaling downstream of TLR2 activation is not affected by TTO. In contrast, terpinen-4-ol and alpha-terpineol inhibited the TLR4- and TLR2/4-induced production of IL-6 (Fig. 2e, f). These differences in the biological activity of the oils according to the nature of stimulation and type of receptor that was preferentially activated may be due to the specific characteristics of signaling by TLR2 and TLR4 in macrophages. Considering that terpinen-4-ol and alpha-terpineol are constituents of TTO, the fact that the modulation of IL-1b and IL-6 by the complete oil (TTO) occurred only after the activation of TLR4 may be due to the influence of other oil components (Fig. 2e, f).
IL-10 is an endogenously produced anti-inflammatory cytokine that possibly acts as a negative regulator of inflammation. Thus, an inflammatory stimulus also induces its negative regulator, as a way to control the process and prevent excessive damage to host tissues. All tested oils and concentrations (except for 0.004 % TTO) significantly decreased the production of IL-10 (Fig. 2g, h), which is consistent with a report by Hart et al. [10] indicating that TTO at 0.125 % reduced the levels of IL-10. However, according to Che´zet et al. [15], TTO at a lower concentration (0.01 %) increased the secretion of IL-10 by human lymphocytes and monocytes stimulated with lectin from Phaseolus vulgaris, suggesting that the cell type, concentration of oil and nature of the stimulus are crucial to the biological activity of TTO. To obtain insight into the mechanisms associated with the modulation of cytokine
production by TTO and its components, we assessed if the oils and its tested components had an effect on the activation of intracellular signaling pathways that were relevant for cytokine expression and also activated by TLR4 and TLR2/4 signaling.
The cytoplasmic portion of TLR2 can interact with two adapter proteins: myeloid differentiation factor 88 (MyD88) and the TIR domain containing the adaptor protein MyD88 (TIR domain-containing adapter-like adapter protein/MyD88 or TIRAP/MAL) [27–29]. Activation of TLR4 can recruit four adapter proteins, in addition to the adapter MyD88 and TIR domain containing the TIRinducing interferon-beta (TIR domain-containing adapter inducing IFN-β or TRIF) and the adapter molecule TRIFrelated (TRIF-related adapter molecule or TRAM) [27–29].
TRIF and TRAM are related to the activation of transcription factors such as NF-κB, MAPK and interferon regulatory factor 3 (interferon regulatory factor 3-IRF3)
[30]. It is possible that TTO operates in TLR4 signaling mainly by a MyD88-independent pathway.
The production of target cytokines by macrophages upon activation of TLR4 and TLR2/4 was dependent on signaling through NF-jB, p38 and ERK MAPK, except for TLR2/4-induced IL-10 production, which was not dependent on ERK activation (Fig. 3).
Our results indicate that TTO and its components can modulate the activation of NF-jB and ERK MAPK a pretreatment before TLR2/4 or TLR4 stimulation (Fig. 5).
Thus, the inhibition of LPS-induced production of IL-1b, IL-6 and IL-10 by the TTO and its components (Fig. 2c–h) probably involves these signaling pathways. The TTO in TLR4 activation decrease the NFKB, ERK and p38, since the activation of TLR2/4 have no effect. Terpinen-4-ol alone modulated the production of ERK by TLR2/4 and TLR4 stimulation, indicating that the action of the oil on ERK is mainly terpienen-4-ol. The Alfa revealed modulation of NF-KB and ERK via TLR2/4 that was not representative of the total oil in the analysis. What we can observe is that terpinen-4-ol is a major asset component the anti-inflammatory capacity of TTO via decrease of ERK when signaling pathways TLR4 is activated.
For the p38 signaling pathway TTO is revealed to be a modulator upon activation of TLR4 alone and this action does not occur by terpinen-4-ol or the alpha since these two oils surprisingly increased production of p38. LPS-induced production of TNF-α (which is also dependent on NF-kB, ERK and p38 MAPK) was not affected by TTO or its components this supports the hypothesis that the biological effects of the tested compounds involves alternative mechanisms.
This study investigates the possible interference of TTO and its components to activation of transcription factors. As the two components of TTO assessed in this study have similar effects in regulating the production of inflammatory cytokines, one would anticipate an additive or synergistic effect by the use of TTO, which contains both terpinen-4-ol and alpha-terpineol, resulting in a potentiation of the regulatory effects. However, concentrations of TTO that contain both terpinen-4-ol and alpha-terpineol simultaneously did not have a more potent biological effect in comparison with the equivalent concentrations (as estimated from the concentrations of these major components in TTO by IOS standards) of each major component used individually. This suggests that in the complex mixture there may be interactions/ interferences between these major compounds or with other components that are present in TTO in lower relative proportions. This finding is relevant and should be considered in future assessments of TTO for therapeutic purposes. In this sense, terpinen-4-ol was shown to be the most potent modulator of LPS-induced cytokine production with significant reductions in the levels of IL-1b, IL-6 and IL-10 via reduction in ERK.
In vivo studies have demonstrated the anti-inflammatory activity of the oil and its constituents, such as reducing contact hypersensitivity after topical application [31, 32], decreasing inflammation-associated edema [16] and reducing redness of the skin [14, 33, 34]. However, no studies to date have assessed the effect of TTO or its major
components in periodontal disease models, which is also a useful model for other conditions associated with chronic inflammation and sustained host–microbe interactions.
In conclusion, we have shown that TTO, and especially, its major component terpinen-4-ol are potent modulators of pro-inflammatory cytokine production by macrophages upon activation of TLR4 and TLR2/4. This effect was mediated by interfering with the NF-κB, p38 or ERK MAPK pathways.
Acknowledgments This work was supported by grants from the Brazilian Federal Government through the National Council for Scientific and Technological Development (CNPq) and Coordination for Improvement of Higher Education Personnel (CAPES). This study was also supported by the State of Sa˜o Paulo Research Foundation (FAPESP) (Grant number 2009/54190-0 and 2010/18968-3).