R&D
Preparation and Properties of Glycerin Ester of Tung Oil Modified Rosin
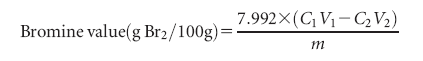
R&D
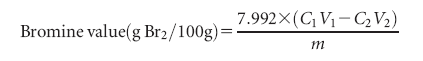
Guozhang Ma,1 Tong Zhang,1,2 Jianbing Wu,1,2 Caiying Hou,1 Lixia Ling,3 Baojun Wang2
1. Shanxi Research Institute of Applied Chemistry, Taiyuan 030027, Shanxi, China
2. Key Laboratory of Coal Science and Technology, Ministry of Education and Shanxi Province, Taiyuan University of Technology,
Taiyuan 030024, Shanxi, China
3. Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
NOTICE: The original research paper is published by Wiley Periodicals, Inc. J. Appl. Polym. Sci. 130: 1700–1706, 2013, visit to the page.
ABSTRACT: Tung oil modified rosins (TR) were prepared by reaction rosin with different amount of tung oil via Diels-Alder addition reaction and further used in the formulation of glycerin ester of tung oil modified rosins (GTR) with flexible characteristics. The effects of the amount of tung oil on the bromine value, molecular weight, thermostability, physical, and tackifying properties of GTRs were studied. The results showed that the bromine value of GTRs decreased and molecular weight of GTRs increased with the increase in the amount of tung oil. Meanwhile, increasing the amount of tung oil resulted in a significant decrease in both the softening points and viscosities of GTRs, but a slight increase in the thermostability of GTRs due to the incorporation of the flexible fatty chains into the rigid hydrophenanthrene units in osins. Applied in PU adhesive as tackifier, the increase of the flexible chains content in GTRs led to an increase first and then decrease in both the miscibility of GTRs with PU and the T-peel strength of adhesives. The elongation at break of films increased monotonously, but their tensile strengths increased first and then decreased with increasing the flexible chains content in GTRs. GTRs with desired properties were prepared when the tung oil/rosin weight ratios were in the range from10/100 to 40/100.
KEYWORDS: addition polymerization; resins; synthesis and processing
Rosin is an abundantly available natural material composed of 90% rosin acids and 10% nonacidic materials. The rosin acids are a mixture of abietic-type acids with a conjugated double bond and pimaric-type acid with nonconjugated double bonds. The presence of a carboxyl group and the conjugated double bond in their structure imparts to them a high chemical reactivity. Therefore, rosin has been extensively used as a raw material for the preparation of various monomers and polymers with specific chemical structures and valuable properties.1
In recent years, more and more efforts have been made to prepare rosin esters or polyesters used as tackifiers for adhesives and rubbers, as film-forming materials for coating and printing inks, as microencapsulating products used in chewing gum and cosmetics.2–14 To increase molecular weight and improve properties of the esters, rosin acids are modified by hydrogenation, dehydrogenation, or formation of Diels-Alder adducts before esterification. Hydrogenation and dehydrogenation of rosin render rosin esters less susceptible to oxidation.2 Diels-Alder adduction reaction of Abietic-type acids in rosin with dienophile substance (acrylic acid, acrylonitrile, maleic anhydride, fumaric acid, itaconic acid, cinnamic acid, and resol phenolic resin) is a commonly used method to synthesize multifunctional monomers in the preparation of vinyl esters,3–5 epoxy esters,6–8 polyesters,9,10 polyesteramides,11 polyesterimides,12 and phenolic resin modified rosin esters,13which exhibit the properties of high chemical reactivity, high softening point, increased thermal, or great hardness. Although rosin esters tend to pose handling problems because of their brittle nature, this drawback can be easily overcome by adding adequate hydrophobic plasticizers such as tributil sebacate and tributil citrate.14
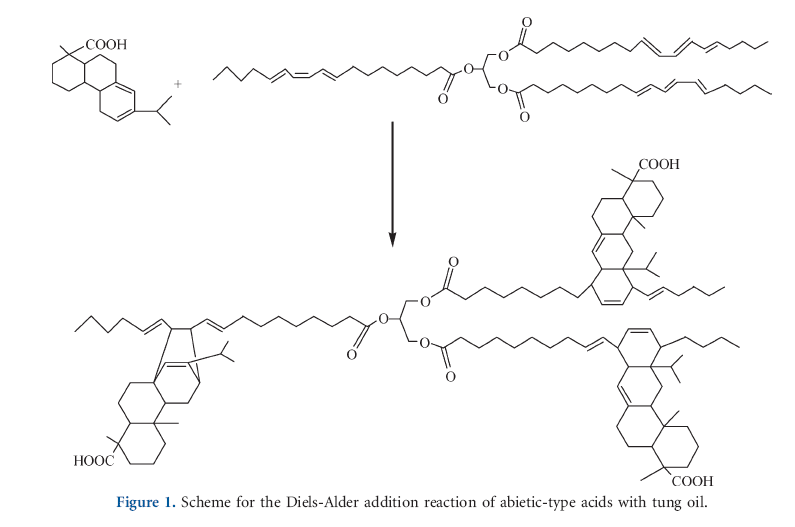
This work aims to prepare rosin ester with flexible characteristics. Tung oil, main constituent a glyceride of eleostearic acid with a conjugated triene structure, is readily available as a major product from the seeds of the tung tree. The high unsaturation and conjugation of the C=C bonds in tung oil makes it a high chemical reactivity with styrene,15 divinylbenzene,16 acrylates,17 or maleic anhydride.18 Therefore, we try to introduce the flexible chains into rosins by modifying rosin acid with tung oil before esterification. The reaction of tong oil and rosin acid was discussed. The thermal and physical properties of glycerin ester of tung oil modified rosins (GTR) were determined and their tackifying properties in polyurethane adhesive were evaluated.
Materials
Tung oil (gravity: 0.9364 at 20ºC; refractive index: 1.519 at 20ºC; bromine value: 108 g Br2/100 g; acid value: 4.2 mg KOH/ g). Gum rosin (melting point: 74ºC; acid value: 162 mg KOH/g, bromine value: 84 g Br2/100 g). It consists of about 73% abietictype acids and 16% pimaric type acids. Isophorone diisocyanate (IPDI), polypropylene glycol (PPG, Mw 5 1000), polyadipate of 1,4-butanediol (PAB, Mw 1000) and isophorone diamine (IPDA). The other chemicals were of analytical grade and purchased locally. PPG and PAB were dried at 120ºC for 2 h under vacuum to remove moisture before use, and the other materials were used without further purification.
Preparation of Glycidyl Ester of Tung Oil Modified Rosin
Rosin (302.2 g; 1 mol) was melted at 170ºC in a 500 mL four-necked flask equipped with a mechanical stirrer, nitrogen purging system, thermometer, and refluxing device, and then a certain amount of tung oil was added slowly into the flask and the mixture was stirred for 4–6 h vigorously at 170ºC under nitrogen atmosphere. After 31.2 g (0.34 mol) of glycerin was added for 1 h in the catalyst of magnesium oxide at the reaction temperature from 180 to 220ºC, the reaction was carried out at 250ºC until the acid value was <18 mg KOH/g. The low fractions (nonacidic material of rosin and moisture) were removed under reduced pressure for 15 min to yield yellowish solid, named GTR. When rosin acid was esterified directly by glycerin, the obtained product was named GR.
Preparation of Polyurethane/GTR Adhesives
In a 1000 mL four-necked flask equipped with a mechanical stirrer, nitrogen purging system, thermometer, and a refluxing device, 50 g (0.05 mol) of PPG and 50 g (0.05 mol) of PDB were reacted with 44.5 g (0.2 mol) of IPDI at 75ºC for 3 h under vigorous stirring. After reaction, 300 mL ethyl acetate was introduced. Then 17.0 g (0.1 mol) of IPDA was added at the same temperature during 5 min under continuous stirring and allowed to react for another 1 h. Finally, 64.6 g of GTR was added, and the solution was stirred for 40 min to obtain homogeneous polyurethane/GTR adhesive, named PU/GTR adhesive. The PU/GTR weight ratio was in proportion to 100/40.
Measurements and Characterization
Acid Number. Acid number was determined with 0.1 mol/L aqueous KOH in the presence of phenolphthalein with an 80/20 mixture by volume of toluene and ethanol as solvents.
Bromine Value. The bromine value was measured by addition
of bromine using the bromate-bromide method.19 The sample was dissolved in a 10/50 mixture of chloroform and acetic acid, further reacted with 0.1 mol/L bromine solution (bromatebromide mixture) for 1 min at room temperature, then added 10% KI solution and titrated with 0.1 mol/L Na2S2O3 solution in presence of starch indicator. Bromine value was calculated from the following equation: where C1 is the concentration of bromine (mol/L), V1 is the addition volume of bromine solution (mL), C2 is the concentration of Na2S2O3 (mol/L), V2 is the consumption volume of Na2S2O3 (mL), and m is weight of sample (g).
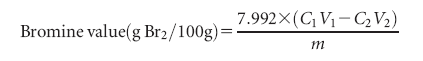
Fourier Transform Infrared Spectroscopy. The infrared spectra were measured using a Nicolet 360 Fourier-transform infrared spectrometer (USA). Rosin or GTR was ground and mixed with KBr to make pellets and tung oil was directly coated on the KBr pellets. Fourier transform infrared spectroscopy (FTIR) spectra were collected with a resolution of 4 cm-1 in the range of 450 and 4000 cm1 and 32 scans was used to reduce the noise.
Gel Permeation Chromatograph. The average molecular weight (Mw), number-average molecular weight (Mn), and molecular weight distribution (Mw/Mn, PDI) of GTRs were determined at 40ºC using gel permeation chromatography (GPC; Waters, USA) equipped with a waters 1515 isocratic HPLC pump and 2410 RI detector. The solvent was tetrahydrofuran at a flow rate of 1.0 mL/min and the polystyrene standards were used for the calibration.
Thermogravimetry. The thermal decomposition of GTRs was determined by a HCT-1 thermal gravimetric analyzer (Henven Scientific Instrument, China). Sample of 6–10 mg was placed in a platinum sample pan and heated from 25 to 800ºC at a heating rate of 20ºC/min. Nitrogen was used as purge gas at flow rate of 50 mL/min.
Softening Point. The softening point of GTRs was measured by a ring and ball method according to ASTM D36-1995. In this test, GTR was melted and cast into a shouldered ring and then heated at a constant rate in a glycerin bath. The temperature at which GTR disks soften and sag downward under the weight of a standard steel ball was noted as the softening point.
Solubility. GTR (1 g) was dissolved in 15 mL organic solvent in a tared 50-mL centrifuge tube at 35ºC under ultrasonic wave for 1 h, and then spun in a centrifuge for 20 min. Solubility was tested according to the amount of precipitation obtained after separated and dried. No precipitation was expressed as “soluble,” partly soluble corresponded to less than 1 g of precipitation and insoluble corresponded to about 1 g of precipitation.
Viscosity. The viscosity of GTR-linseed oil solution was tested by a NDJ-79 rotation viscometer (Shanghai Scientific Instrument, China) at 25ºC with the spindle speed of 60 rpm. The solution was obtained by dissolving GTR in refined linseed oil at 150ºC according to the weight proportion of 1 : 1.
Evaluation of adhesive properties
Preparation of Adhesive Films. PU/GTR adhesives were cast into glass molds and dried at room temperature for 48 h. After the ethyl acetate evaporated, the cast films were dried at 60ºC for 1 h under vacuum. The resulting thickness of adhesive layer was measured using a thickness indicator and thick sheet specimens with a thickness of about 2 mm were obtained.
Visual Observation. Solutions of PU/GTR adhesives in 50 mL tubes were maintained at 25ºC for 24 h and visually observed to see whether they were transparent or opaque at this temperature.20 Films obtained above were placed in a vacuum oven at 25 and 50ºC for 3 h to observe their transparent performances, respectively.
Mechanical Properties. The mechanical properties of adhesive films were measured at room temperature with a LCD electronic tensile meter according to ASTM D 638 specifications. A crosshead speed of 50 mm/min was used throughout these investigations to determine the ultimate tensile strength and elongation at break for all samples. The quoted values were the averages of five measurements.
T-Peel Strength. The T-peel strength of PU/GTR adhesive was measured in the above tension meter. The PVC test samples had a dimension of 30×150×5 mm. Certain amount of adhesive was applied to each PVC strip and the strip was left to dry for 1 h. After evaporation, solid PU/GTR adhesive films were formed on the PVC strips and were heated to 80ºC using infrared lamps. The two melted PU/GTR films on the PVC strips were immediately placed in contact and a pressure of 0.8 MPa was applied for 10 s to achieve a suitable joint. Finally, the immediate T-peel strength (obtained 5 min after joint formation) and final T-peel strength (obtained at 24 h) were obtained after measuring at a peel rate of 250 mm/min. The values obtained were also the average of five replicates.
Preparation and Characterization of GTRs
The present work deals with the preparation of tung oil modified rosin acids (TR) and esterification of them by glycerin. The esterification of rosin acids or their derivates is known as a common and mature reaction, but there are few reports on the reaction of rosin acid and tung oil.21 Containing conjugated double bond, abietic-type acids in rosin or tung oil is a diene, which can react with dienophile or dimerize itself by the Diels-Alder addition reaction. However, they are not good dienophile due to their chemical stereo-hindrance; therefore, dimerization of rosin acid or tung oil must be carried out in presence of Lewis acid catalyst under elevated temperatures.22,23 In this study, to introduce the desired content of flexible chains into rosin acids, different amount of tung oil were used to prepare TR via Diels-Alder addition reaction without catalyst. Because the excess mole of rosin was used during modification in this study, TR was a mixture of rosin acids and their derivates. Although the conversion of the s-trans to s-cist conformation by the rotation of carbon-carbon single bond between the conjugate systems is possible under thermal process, the reaction undergo between abieti-type acid and tung oil with cis conformation.18 The reaction scheme was selected and represented in Figure 1.
The corresponding reactions are characterized by the FTIR spectra showed in Figure 2. In the FTIR spectrum of the rosin (curve a), a broad absorption bands in the range 2300–3500 cm-1 is specific to OH stretching absorption of carboxylic acid and aliphatic carbon-hydrogen absorption due to the hydrogen bonding of rosin acid, meanwhile, a absorption peak at 2651 cm-1 appears for hydrogen bonding and the peak for carbonyl group locates in lower frequency at 1694.2 cm-1.10,11 The FTIR spectrum of the tung oil (curve b) shows a sharp shoulder peak at 3014 cm-1 and a small peak at 1643 cm-1 corresponding to unsaturated C-H and C=C bonds respectively.The existence of peaks at 965 and 735 cm-1 indicates that both cis and trans conjugated unsaturations of the eleostearic chains exist in tung oil. The peak at 1745 cm-1 is assigned to the ester carboxylic group in triglyceride molecules.24
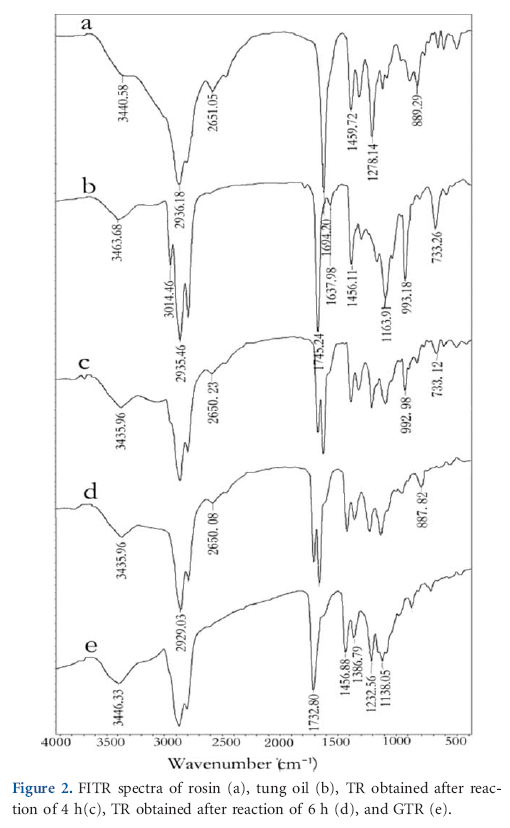
Figure 2(c,d) are the FTIR spectra of TR obtained in 4 and 6 h at 170ºC respectively. Each absorption peak, which was changing its intensity, was compared with the standard absorption peak of 1745 cm-1 from C=O group of tung oil. When reaction was carried out for 4 h, it is observed the decrease in the intensity of the absorption peaks at 3010, 965, and 735 cm-1 and disappearance in the peak at 1643 cm-1 corresponding to unsaturations. After 6 h reaction, all of these peaks disappeared. However, there were hardly any changes in the other peaks no matter how long time the reaction extended.
FTIR analysis was used to confirm the esterification of TR with glycidyl according to the preparation procedure. In Figure 2(e), it was observed in the disappearance of all of peaks corresponding to unsaturations and two sharp bands at 2651 and 1694.20 cm-1 for carboxylic group, meanwhile, the intensity of peak at 1732.08 cm-1 for ester carbonyl group increased. The spectra of glycerin ester of TRs obtained at 4 and 6 h were almost the same. It indicated that Diels-Alder addition reaction between tung oil and rosin continued while esterification of TR. To shorten the reaction time, the modification time of rosin by tung oil was within 4 h.
The decrease in iodine or bromine value of reactants corresponds to the degree of the Diels-Alder addition reaction.22 Therefore, the change of unsaturation in GTRs and their raw materials were analyzed by bromine value analysis, and the result is shown in Figure 3. The bromine value of GR was almost the same as that of rosin, but the bromine value of GTRs decreased with an increase in the amount of tung oil. The results of bromine analysis showed that the modification of rosin by tung oil was based on Diels-Alder addition reaction.
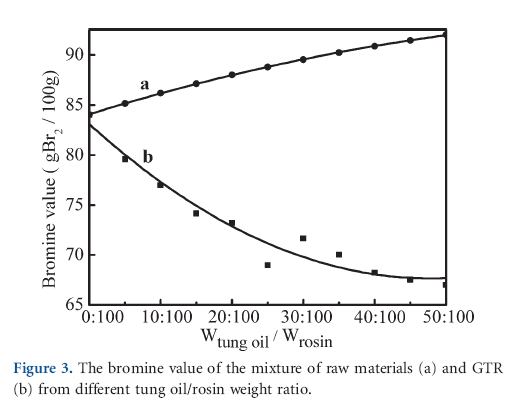
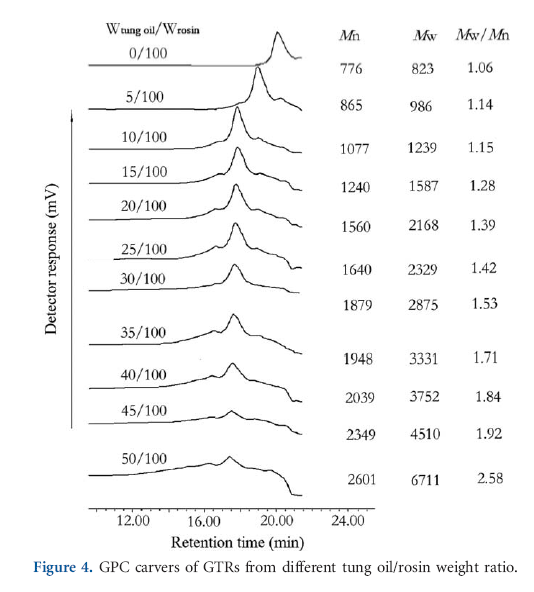
Figure 4 illustrates the effect of the amount of tung oil on the molecular weight and PDI of GTRs. The average molecular weight (Mw) of GR was 823 g/mol and its PDI was 1.06. However, the Mw of GTRs increased and their PDI broadened progressively with increase in amount of tung oil. Besides the main peak, a small peak also appeared at a relatively higher molecular weight region after the tung oil/rosin weight ratio increased to 15/100. While their weight ratio reached to 50/100, the Mw and PDI of GTR increased to 6711 g/mol and 2.58, respectively. The reason is that GR was the triglyceride of rosin acid, which had a certain molecular weight, but GTRs were the triglyceride of a mixture of rosin acids and their derivates. Increase the amount of tung oil resulted in the increase in the content of Diels-Alder adducts in the complex mixture of TR, therefore, the molecular weight of GTRs increased and their PDI broadened.
Thermal and Physical Properties of GTR
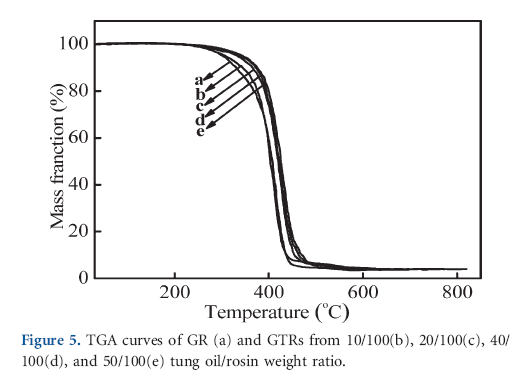
The thermal behaviors of GTRs were evaluated by the TGA experiments, and the results are shown in Figure 5. It can be seen that the weight loss of GR occurred in the range from 212 to 493ºC, which corresponded to the scission of the ester linkage and the hydrophenanthrene structure of rosin.25 However, a slight increases of decomposition temperature were observed after rosin acid modified with tung oil. Increasing in the amount of tung oil resulted in an increase in the decomposition temperature of GTRs. The increased thermostability of GTRs can be ascribed to the simultaneous action of some factors as follows: the decrease in the content of vulnerable conjugated double bonds from rosin acids and the formation of extended rings by the Diels-Alder addition reaction; the increase in the molecular weight and the introduction of flexible fatty chains into GR.10
The softening point is also an important property of rosin ester.1 As illustrated in Figure 6, the softening points of GTRs depended mainly on the tung oil/rosin weight ratio. Increase in the amount of tung oil led to the low in the softening points of GTRs. When tung oil/rosin weight ratio reached to 50/100, GRT was close to sticky solid, which pose handling problems during its usage.
The widely accepted fact is the increase of molecular weight of polymer resulting in its high softening point. However, the opposite results were due to the special chemical structure of GTRs, which contained two types segments. One of the segments was rigid multirings in rosin and new formed during Diels-Alder cycloaddition reaction, which attributed to the high softening point. The other was the flexible saturated or unsaturated straight-chains, which tended to coil and resulted in low softening point because of their “volume effect” or “solvent effect” on the polymers.26,27
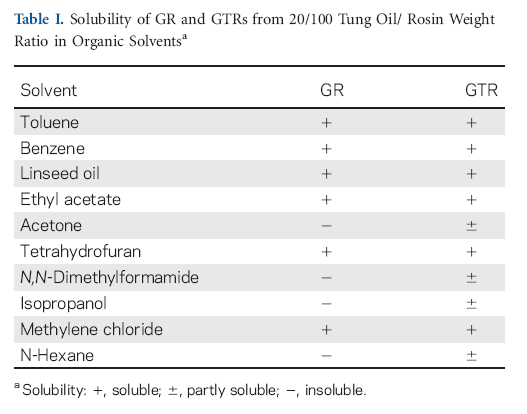
The solubilities of GR and GTR obtained at 20/100 tung oil/ rosin weight ratio were tested in different organic solvents and the results are given in Table I. GR can dissolve in toluene, linseed oil, ethyl acetate, tetrahydrofuran, and methylene chloride. However, GR exhibited poor solubility in acetone, N,N-dimethylformamide, isopropanol, and n-heptane. After modification with tung oil, the solubilities of GTR in above solvents improved greatly because GRT contained flexible chains, which can increase its solubility in organic solvents.28
The intrinsic viscosity is a quantity characteristic of a polymer.13 Figure 7 shows the viscosities of GTRs in linseed oil solution determined at 25ºC. The same concentration of GTRs exhibited different viscosities. Increase in the amount of tung oil led to the low in the viscosity of GTRs solution. Generally, the solution of polymer with higher molecular weight has higher viscosity. However, the structure of polymer also affects the viscosity of polymeric solution. The viscosity is high for the polymer with rigid chains and low for the polymer with flexible chains in the same kind solution.28,29 Increasing the content of flexible chains in GTRs led to a decrease in the viscosities of GTRs solution although the their molecular weight increased.
Tackifying Properties in Polyurethane Adhesive
Thermoplastic polyurethane elastomers are used as adhesives in the automotive, footwear, and furniture industry. These adhesives show excellent performance but lack of immediate adhesion, and addition of rosin esters as tackifier is necessary to produce adequate bond to PVC and other substance.30 However, the addition of external tackifiers generally produces poor miscibility with PU and a decrease in adhesion is also obtained.31 In this study, GTRs were incorporated into PU solution to evaluate their tackifying properties. The determination results of the transparent performances of solutions and films, mechanical properties of films and T-peel strength of adhesives are given in Table II.
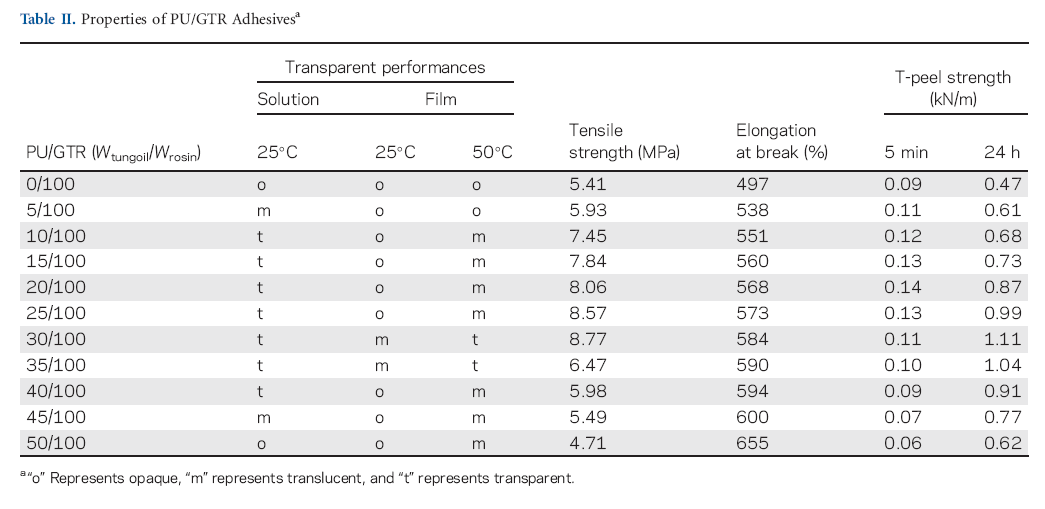
To examine the PU/GTR miscible extent, the transparent performances of adhesive solutions and films were visually observed. The adhesive solution was opaque when GR was used as tackifier. However, the adhesive solutions turned to transparent progressively with the increase in the flexible chains content in GTRs. When GTR prepared with 45/100 weight ration, its adhesive solution became translucent again. The same trend was observed in the transparent performances of adhesive films. Adhesive films from GTRs prepared with 30/100 and 35/100 tung oil/rosin weight ratios were translucent at 25ºC, but the other adhesive films were opaque at same temperature. When the films were maintained at 50ºC, the adhesive films from GT and GTR prepared with 5/100 tung oil/rosin weight ratio still remained in opaque performance, whereas transparency change of the other films were observed. The transparence of adhesives contributed to the miscibility of GTRs and PU.20 High flexible chains content in GTRs resulted in producing good miscibility with PU which has a linear segmented structure constituted by soft flexible segments and a few hard segments,30 but too many content of flexible chains in GTRs make it impossible to gain desired properties.31 Therefore, good miscibility of PU and GTRs were obtained when GTRs prepared with the tung oil/rosin weight ratio in the range from 10/100 to 40/100.
The elongation at break of films increased monotonously, but their tensile strengths increased first and then decreased with increasing the flexible chains content in GTRs. The results indicated GTRs can improve the mechanical properties in both elongation at break and tension strength of adhesive films. When GR was used as tackifier, the mechanical behavior of film was poorer due to the worse miscibility of PU and GR with hydrophenanthrene structure. An increase in the flexible chains content in GTRs led to a higher elastomeric character expressed by elongation at break of films. However, appropriate content of flexible chains in GTRs resulted in higher tension strength of the films, and too many content of flexible chains in GTRs increased the elastomeric character but deceased tension strength of the films.
The T-peel strengths of adhesives at 5 min and 24 h increased firstly and then decreased with increase in the flexible chains content in GTRs. The highest T-peel strength of adhesive at 5 min were obtained when GTR prepared with 20/100 tung oil/rosin weight ratio, and a slight difference was that adhesive had best adhesion at 24 h when GTR prepared with 30/100 tung oil/rosin weight ratio. The reason was that suitable soft/hard segments ratio in the adhesives resulted in their good adhesion. For the PU/GTR adhesives, the increased tung oil/rosin weight ratio resulted in the increased soft segments content in GTRs, which caused the high crystallinity of soft segments in adhesive.32 Therefore, T-peel strength of adhesive increased with the increase in the flexible chain content in GTRs. However, too many soft segments content in GTRs were also unfavorable to the adhesion properties. In this point, GTRs had excellent tackifying properties in PU when they were prepared by the tung oil/rosin weight ratio from 10/100 to 40/100.
Tung oil was used to modify rosin via Diels-Alder addition reaction above 170ºC without catalyst. The modified rosin was esterified with glycerin to produce GTRs with flexible characteristics. Increasing the amount of tung oil resulted in the increase of molecular weight and wide PDI of GTRs. Meanwhile, the softening points and viscosity of GTRs decreased and their thermostability increased with the increase in amount of tung oil.
The amount of tung oil also affected the tackifying properties of GTRs in PU adhesives. Both the miscibility of GTRs with PU and T-peel strength of adhesives (at 5 min and 24 h respectively) increased firstly and then decreased with the increase in the flexible chains content in GTRs. An increase in the flexible chain content led to an increase in the elongation at break of films, but the tensile strength of films increased firstly and then decreased with the increase in the flexible chain content. The desired adhesion properties were obtained when GTRs prepared by the tung oil/rosin weight ratio from 10/100 to 40/100.
This study was supported by Shanxi Scholarship Council of China (2012-8), Scientific Research Foundation of Shanxi Province, China (No. 20111101059), and Youth Natural Science Foundation of Shanxi Province, China (No. 2010021013-2), which are gratefully acknowledged.