Industry News, Adhesives & Sealants & Tackifiers
Liquid Polysulfide Polymers for Chemical- and Solvent-Resistant Sealants

Industry News, Adhesives & Sealants & Tackifiers

Liquid polysulfide polymers are the oldest specialty elastomer. They are characterized by solvent and chemical resistance, great electrical properties, low-temperature flexibility, oxygen and ozone resistance, high impermeability to many gases and moisture, good adhesion properties, and flex-crack resistance.
These low- to high-viscosity polymers have highly reactive terminal thiol or epoxy groups and are widely used as sealants for insulating glass windows, sealants in construction, sealants for aircraft fuel tanks and fuselages, epoxy modifiers, or intumescent protective coatings.
Liquid polysulfide polymers were re-discovered in 1926 by Joseph C. Patrick and Nathan Mnookin when they were trying to react a,v-alkyl halides with inorganic polysulfides (e.g., Na2Sx, x = 4-6). The inventors created high-molecular-weight rubbers with outstanding chemical resistance against solvents, gasoline, ultraviolet (UV) light and ozone.
Liquid polysulfide polymers are basically short-chain polymers with two consecutive sulfur atoms in the polymer backbone. They are terminated by highly reactive thiol groups.
The predominant monomer in the manufacture of liquid polysulfide polymers is Bis-(2-chloroethyl-) formal (diformal) and sodium-polysulfide (Na2Sx) with a sulfur x-rank of 2.4 to 2.6. 1,2,3-Trichloropropane (TCP) is added during the polymer synthesis to control the degree of branching of the polymer backbone. Polymerization of mentioned monomers to polysulfide polymers takes place in environmentally friendly water-based dispersion under the conditions of an emulsion polymerization according to the scheme in Figure 1.
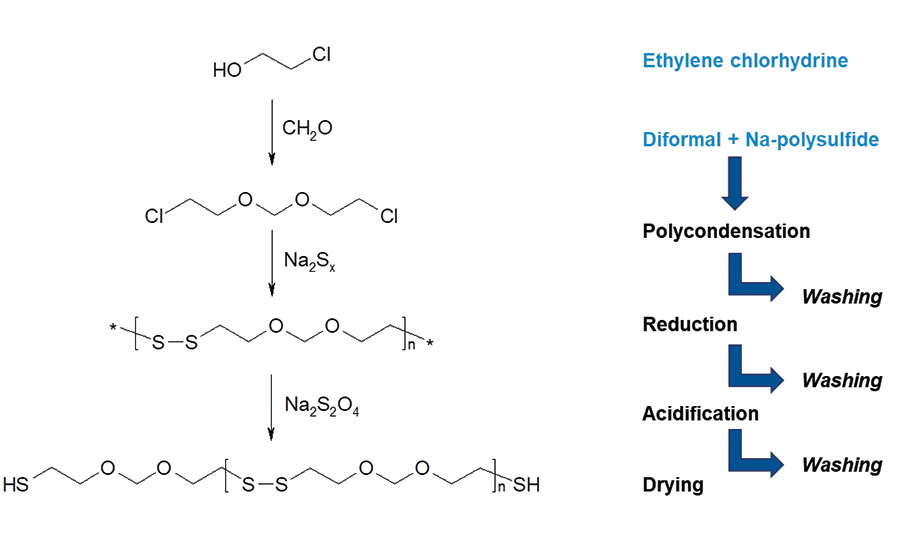
Figure 1. Emulsion polymerization. ©ASI
The molecular weight of different polysulfide polymers grades ranges from 1,000 to approximately 7,000 g/mol.
Lower-molecular-weight polymers are used as reactive diluents in classical sealant applications and as modifier polymer and curing partner to epoxy resins improving the flexibility of the resulting adhesive. The most common grades of polysulfide polymers have molecular weights of 2,000-7,000 g/mol and are widely used in highly flexible sealants in insulating glass, as well as construction and aerospace applications.
During synthesis of polysulfide polymers, crosslinked polymer latex with a molecular weight of approximately 300,000 g/mol is formed. In subsequent steps, the high-molecular-weight solid polysulfide polymer latex gets chemically reduced to the required chain length, molecular weight and SH-content of the targeted liquid polysulfide polymer by using reductive splitting agents such as Na2SO3, Na2S2O4 and NaSH (see Figure 2).
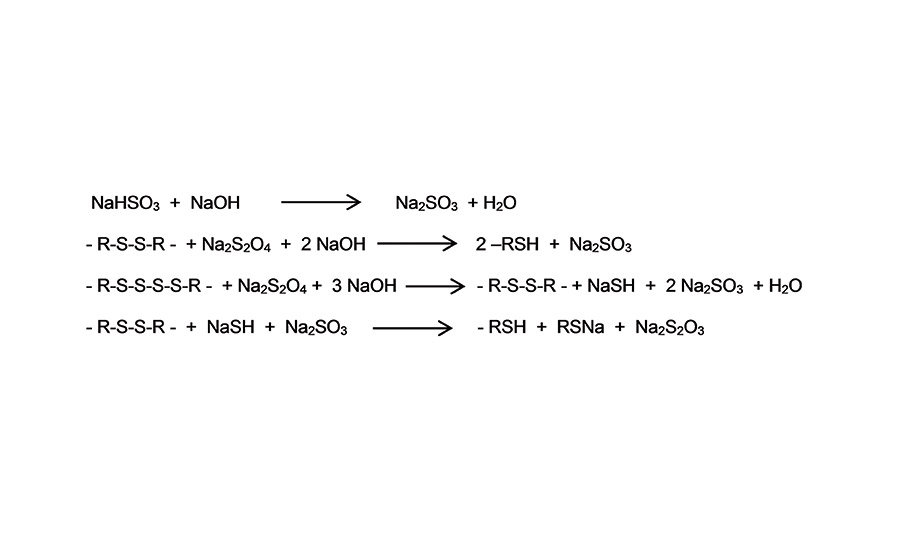
Figure 2. Illustration of reductive splitting agents. ©ASI
The general polymer backbone structure of polysulfide polymers is roughly described by the following structural formula:
The resulting polymer has an aliphatic polymer backbone structure with low-temperature flexibility down to approximately -55°C. In addition, it provides chemical resistance to solvents, oils, fuels, and bases of all commercially available polymers due to its high sulfur content.
Liquid polysulfide polymers are light brown-colored, medium- to high-viscosity polymers. The polymers have been commercialized in a range of different viscosities, molecular weights, sulfhydryl (SH) contents and branching (see Table 1). The average molecular weight as a measure of chain length, SH-content and degree of branching determines the viscosity.
| Type of liquid polysulfide polymers | Unit | G10 | G112 | G131 | G1 | G12 | G21 | G22 | G44 | G4 |
| n | 26-27 | 23-25 | 30-38 | 20-21 | 24-27 | 13-16 | 14-18 | <7 | <7 | |
| Avg mw. | g/mol | 4400-4700 | 3900-4400 | 5200-6500 | 3400-3600 | 4100-4600 | 2100-2700 | 2400-3100 | <1100 | <1100 |
| SH-content | % | 1.4-1.5 | 1.5-1.7 | 1.0-1.3 | 1.8-2.0 | 1.5-1.7 | 2.5-3.1 | 2.1-2.7 | >5.9 | >5.9 |
| Crosslinking agent | mol-% TCP | 0 | 0.5 | 0.5 | 2.0 | 0.2 | 2.0 | 0.5 | 0.5 | 2.0 |
Liquid polysulfide polymers are soluble in solvents like benzene, toluene, and dichlorethane, and are partially soluble in aliphatic esters and ketones. Plasticizers, such as different phthalates, benzoates or chlorinated paraffins, are chemically compatible with liquid polysulfide polymers. There is no chemical compatibility to water or aliphatic hydrocarbons. Dispersed sulfur is chemically added to the polymer, generating S3 or S4 structures, and then receives part of the polymer backbone. Due to the highly reactive thiol end-groups, polysulfide polymers react under the influence of oxidizing agents, epoxies, isocyanates or acrylates by forming highly elastic and rubber-like high-molecular-weight macromolecules without significant physical shrinking.
Curing of polysulfide polymers to high-molecular-weight elastomers is most widely done by oxidizing the terminal thiol groups to disulfides. The most commonly used curing agents are oxygen donors, such as lead dioxide, manganese dioxide, and calcium peroxide, and organic hydroperoxides like cumene hydroperoxide.
Theoretically, the polysulfide polymer converts from 2RSH to R-SS-R regardless of the metal dioxide used. In practice, each metal dioxide takes the polymer to different levels of cure. It has been found that PbO2 yields a lower modulus product than MnO2, for example. Curatives with a stronger oxidation characteristic, such as permanganates or dichromates, yield a very high modulus sealant with improved thermal resistance. Organic peroxides tend to yield high-modulus elastomeric polymers.
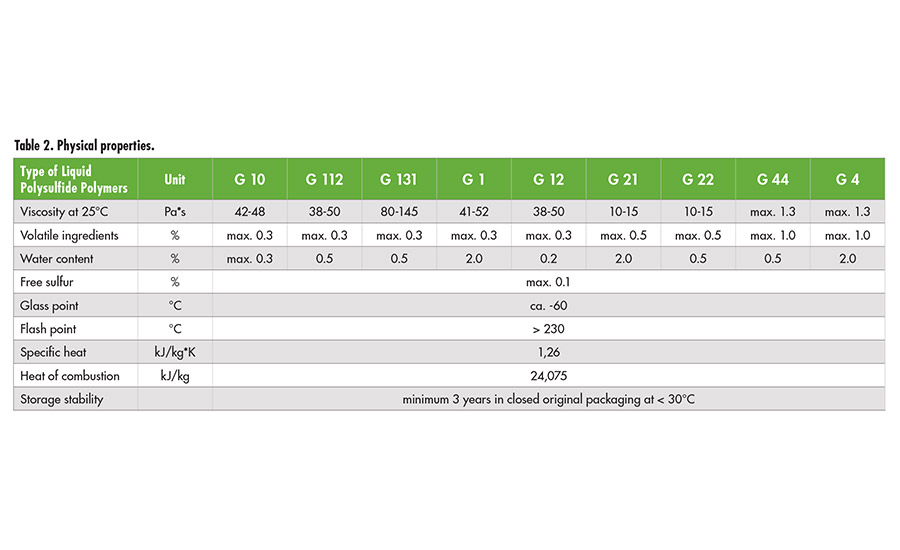
Table 2. Physical properties. ©ASI
Water takes an essential part in the cure reaction of liquid polysulfide polymers. Most reactions can be permanently or severely retarded by the removal of trace water. Zeolites and BaO have been found as water scavengers and are used in the design of water-activated single-component sealant formulations.
In two-component sealants, a minimum level of 0.1-0.5 wt% water is mandatory in the MnO2-based curative part to initiate the curing. It is interesting that BaO, which is used to remove water to retard curing during the preparation of single-pack systems, also acts as a catalyst. In this case, BaO attracts water and creates a basic environment to catalyze the cure once sufficient water is present.
Other influential additives include acids and bases. All the reactions involving RSH can be catalyzed by the addition of a base; conversely, it can be retarded by the addition of acid. In practice, amines and similar compounds are used as catalysts, while weak acids such as stearic or oleic acids are used as retarders. It has been found that organic (mono) mercaptans, used as chain stoppers to reduce the amount of crosslinking, can have a severe retardation effect on curing even at a very low content. MnO2 is relatively insensitive to the mixing ratio, thus providing a special advantage. Traces of water have a strong catalytic effect on the curing speed. Curing speed increases as water is released during the oxidation of the thiol groups as a kind of autocatalytic reaction.
Stoichiometric controlled chemical reactions of polysulfide polymers are possible with epoxies, phenolic resins and isocyanates. The thiol end groups of polysulfide polymers are highly reactive to di-isocyanates, such as 1,3-toluene di-isocyanate (m-TDI), diphenylmethane-4,4´-di-isocyanate (MDI) or multifunctional liquid isocyanates. Using polysulfide polymers instead of common hydroxy-terminated polymers brings the physical advantages of polysulfides to the cured product. Thus, good chemical and solvent resistance, weatherability, adhesion, etc., can be attained. In addition, isocyanate-cured systems have advantages over oxidative-cured systems, such as improved adhesion to plastic substrates.
| Curing Agent | Amount in g/100 g liquid polysulfide polymer |
| Manganese (IV) dioxide | 3.9 × SH [%] |
| Calcium peroxide | 4.3 × SH [%] |
| Sodium perborate monohydrate | 2.1 × SH [%] |
| Cumene hydroperoxide/t-butyl hydroperoxide | 4.6 × SH [%] |
| Lead dioxide | 4.7 × SH [%] |
At elevated temperatures, phenolic resins are cured with polysulfide polymers through a polycondensation reaction. The product may be considered as a block copolymer of the rigid phenolic resin and the flexible polysulfide. Polysulfide polymers are widely used as reactive diluents and as curatives in two-component adhesives in blend with primary or secondary amines to cure bisphenol A/F or novolac-based epoxies.
In this case, the polysulfide polymers act as “soft part” and the epoxy resin as “hard part” in the resulting cured flexible adhesive. This results in adhesives with high flexibility, high impact strength, excellent chemical resistance and good adhesion.
Polysulfide polymers are compounded with a variety of plasticizers, reinforcing agents like silica and calcium carbonate, and polymer extenders. Processing the liquid polysulfide polymers in sealants and adhesives requires the use of high-shear mixing equipment.
The cured sealants based on the polymers have excellent resistance to many hydrocarbons, solvents and chemicals. Sulfur is the obvious distinguishing feature of the polymer backbone.
Swelling in solvents is directly related to the degree of crosslinking in the final cured liquid polysulfide polymer. Exceptional good swelling resistance of polysulfide polymers is noticed in hydrocarbons, gasoline, jet fuel, esters, ethers and ketones.
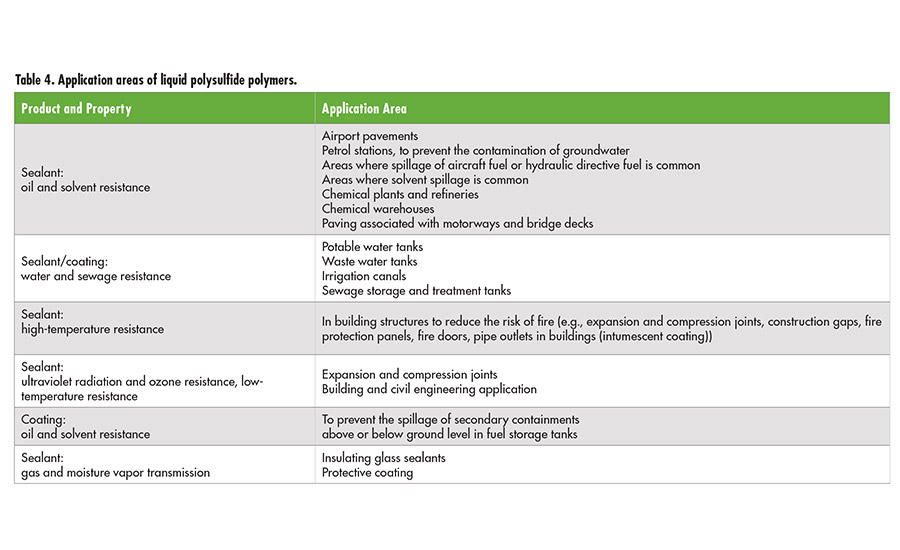
Table 4. Application areas of liquid polysulfide polymers. ©ASI
In addition, polysulfide polymers show excellent low-temperature flexibility, high oxygen and ozone resistance, impermeability to gases and moisture, and excellent flexibility and crack resistance. The formal group as part of the polymer backbone is responsible for this low-temperature flexibility. The absence of carbon-carbon double bonds and aromatic structure in the polymer backbone significantly reduce the sensibility against oxygen, ozone, and UV.
The use of bis-(2-chloroethyl) formal as the predominant monomer in the manufacture of polysulfide polymers results in the formation of polymers with very good low-temperature properties. The glass-transition temperature of these polysulfide polymers is at -55°C. In addition, these polymers do not have a tendency to crystalize above the glass-transition temperature.
Polysulfide polymers are unique in their ability to internally relieve stress even in the cure state by interchange reactions between thiol terminal groups and disulfide linkages. The rate of stress relaxation depends on temperature, UV light or catalysts, especially basic inorganic salts such as NaSH, NaOH, and primary or secondary amines. The process is energized by the strain energy in the vicinity of two polymer disulfide groups.
The energy of activation for this interchange process is at approximately 100 kJ/mol for polymers with S2, S3 or S4 structures in the polymer backbone. The observed stress relaxation may result from trace quantities of S3 or S4 structures in the polymer backbone.
Different polysulfide polymers are mainly used as the base polymer in highly elastic sealants. In aerospace applications, liquid polysulfide-based sealants are basically used to seal all parts of the aircraft fuselage, including fuel tanks. Other applications include high-quality sealants for buildings, water canals, sewage plants, gasoline stations, tank containers for fuel and solvents, highway and airport constructions, concrete coatings and sealants, boat hulls and decks, gaskets, gas-meter diaphragms, and printing rolls. Further important application areas include the use of polysulfide polymers as epoxy and rubber modifiers to improve the flexibility and elongation at break and the use in intumescent protective coating for steel constructions. Alternatively to the SH terminated Thioplast G types, epoxy functionalized liquid polysulfide polymers, Thioplast EPS, might be used. The polymer backbones of this kind are consisting of S2 linkages, formal groups and highly reactive epoxy-end groups.
The polymer backbone of the liquid polymer is dominated by the polysulfide backbone with S2-backbone structure, formal groups and highly reactive epoxy-end groups.
The polymers might be cured by typical hardeners for epoxies such as aliphatic amines, aromatic amines, amido amines, cycloaliphatic amines and Mannich bases. They might be used alone or in combination with other common epoxy resins of the bisphenol A/F or novolac type to formulate protective coatings with excellent chemical resistance and high flexibility.
Sulfur-containing polymers, such as liquid polysulfide polymers, have a characteristic odor. Toxicity tests conducted on representative polysulfide polymers grades indicate that they are not eye or skin irritants, do not cause allergic skin reactions, and have no oral toxicity (LD50 > 5 g/kg). Tests on the lower-molecular-weight polysulfide polymers products show similar findings. Liquid polysulfide polymers are classified as nonhazardous under the criteria set forth in the Hazard Communication Standard (29 CFR 1910.1200) by the U.S. Occupational Safety and Health Administration (OSHA).